Simulating the enzymes of ganglioside biosynthesis with Glycologue
- PMID: 33828618
- PMCID: PMC8008095
- DOI: 10.3762/bjoc.17.64
Simulating the enzymes of ganglioside biosynthesis with Glycologue
Abstract
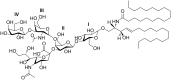
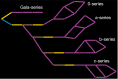
Gangliosides are an important class of sialylated glycosphingolipids linked to ceramide that are a component of the mammalian cell surface, especially those of the central nervous system, where they function in intercellular recognition and communication. We describe an in silico method for determining the metabolic pathways leading to the most common gangliosides, based on the known enzymes of their biosynthesis. A network of 41 glycolipids is produced by the actions of the 10 enzymes included in the model. The different ganglioside nomenclature systems in common use are compared and a systematic variant of the widely used Svennerholm nomenclature is described. Knockouts of specific enzyme activities are used to simulate congenital defects in ganglioside biosynthesis, and altered ganglioside status in cancer, and the effects on network structure are predicted. The simulator is available at the Glycologue website, https://glycologue.org/.
Keywords: Glycologue; Svennerholm nomenclature; gangliosides; glycosyltransferases; neuropathy.
Copyright © 2021, McDonald and Davey.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
