miR-135a Reduces Osteosarcoma Pulmonary Metastasis by Targeting Both BMI1 and KLF4
- PMID: 33828977
- PMCID: PMC8019936
- DOI: 10.3389/fonc.2021.620295
miR-135a Reduces Osteosarcoma Pulmonary Metastasis by Targeting Both BMI1 and KLF4
Abstract
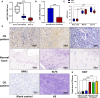
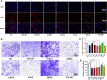
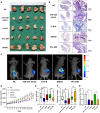
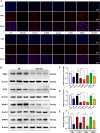
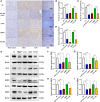
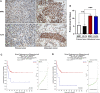
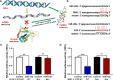
Because of the modest response rate after surgery and chemotherapy, treatment of osteosarcoma (OS) remains challenging due to tumor recurrence and metastasis. miR-135a has been reported to act as an anticarcinogenic regulator of several cancers. However, its expression and function in osteosarcoma remain largely unknown. Here, we reported that abridged miR-135a expression in OS cells and tissues, and its expression is inversely correlated with the expression of BMI1 and KLF4, which are described as oncogenes in several cancers. Ectopic expression of miR-135a inhibited cell invasion and expression of BMI1 and KLF4 in OS cells. In vivo investigation confirmed that miR-135a acts as a tumor suppressor in OS to inhibit tumor growth and lung metastasis in xenograft nude mice. BMI1 and KLF4 were revealed to be direct targets of miR-135a, and miR-135a had a similar effect as the combination of si-BMI1 and si-KLF4 on inhibiting tumor progression and the expression of BMI1 and KLF4 in vivo. Altogether, our results demonstrate that the targeting of BMI1/KLF4 with miR-135a may provide an applicable strategy for exploring novel therapeutic approaches for OS.
Keywords: BMI1; KLF4; metastasis; microRNA; osteosarcoma.
Copyright © 2021 Chen, Mao, Cheng, Jiao, Zhou, Ren, Wu, Lv, Sun and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Long noncoding RNA DANCR knockdown inhibits proliferation, migration and invasion of glioma by regulating miR-135a-5p/BMI1.Cancer Cell Int. 2020 Feb 18;20:53. doi: 10.1186/s12935-020-1123-4. eCollection 2020. Cancer Cell Int. 2020. PMID: 32099526 Free PMC article.
-
Down-regulation of Krüppel-like factor-4 by microRNA-135a-5p promotes proliferation and metastasis in hepatocellular carcinoma by transforming growth factor-β1.Oncotarget. 2016 Jul 5;7(27):42566-42578. doi: 10.18632/oncotarget.9934. Oncotarget. 2016. PMID: 27302923 Free PMC article.
-
Silencing lncRNA ZFAS1 or elevated microRNA-135a represses proliferation, migration, invasion and resistance to apoptosis of osteosarcoma cells.Cancer Cell Int. 2019 Dec 3;19:326. doi: 10.1186/s12935-019-1049-x. eCollection 2019. Cancer Cell Int. 2019. PMID: 31827400 Free PMC article.
-
miR-135a Targets SMAD2 to Promote Osteosarcoma Proliferation and Migration.J Oncol. 2022 Apr 13;2022:3037348. doi: 10.1155/2022/3037348. eCollection 2022. J Oncol. 2022. PMID: 35466322 Free PMC article.
-
The Fas/FasL Signaling Pathway: Its Role in the Metastatic Process and as a Target for Treating Osteosarcoma Lung Metastases.Adv Exp Med Biol. 2020;1258:177-187. doi: 10.1007/978-3-030-43085-6_12. Adv Exp Med Biol. 2020. PMID: 32767242 Review.
Cited by
-
Krüppel-like factors family in health and disease.MedComm (2020). 2024 Sep 10;5(9):e723. doi: 10.1002/mco2.723. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39263604 Free PMC article. Review.
-
Self-Renewal and Pluripotency in Osteosarcoma Stem Cells' Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers.Int J Mol Sci. 2023 May 7;24(9):8401. doi: 10.3390/ijms24098401. Int J Mol Sci. 2023. PMID: 37176108 Free PMC article. Review.
-
Tocilizumab (monoclonal anti-IL-6R antibody) reverses anlotinib resistance in osteosarcoma.Front Oncol. 2023 Jun 19;13:1192472. doi: 10.3389/fonc.2023.1192472. eCollection 2023. Front Oncol. 2023. PMID: 37404767 Free PMC article.
-
DDTC-Cu(I) inhibits human osteosarcoma cells growth by repressing MET/PI3K/AKT signaling pathway.Sci Rep. 2025 Jul 1;15(1):21780. doi: 10.1038/s41598-025-06748-6. Sci Rep. 2025. PMID: 40594499 Free PMC article.
-
Cisplatin and doxorubicin chemotherapy alters gut microbiota in a murine osteosarcoma model.Aging (Albany NY). 2024 Jan 16;16(2):1336-1351. doi: 10.18632/aging.205428. Epub 2024 Jan 16. Aging (Albany NY). 2024. PMID: 38231481 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

