Exosomal miR-130b-3p Promotes Progression and Tubular Formation Through Targeting PTEN in Oral Squamous Cell Carcinoma
- PMID: 33829013
- PMCID: PMC8019696
- DOI: 10.3389/fcell.2021.616306
Exosomal miR-130b-3p Promotes Progression and Tubular Formation Through Targeting PTEN in Oral Squamous Cell Carcinoma
Abstract
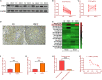
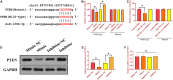
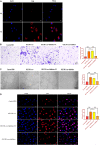
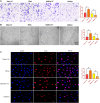
Oral squamous cell carcinoma (OSCC), accounting for two-thirds of head and neck cancer, is characterized by poor prognosis and a high rate of mortality. Exosomes have emerged as potential molecule-shuttle in intercellular communication, thereby regulating the physiological processes of recipient cells. To date, the effect of exosomal microRNAs (miRNAs) on the progression of OSCC has not been fully investigated. In this study, we found that the protein, but not mRNA expression of Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was decreased in OSCC. The results revealed that miR-130b-3p was an important negative regulator for PTEN expression. Additionally, overexpression and knockdown of miR-130b-3p enhanced and inhibited angiogenesis in human umbilical vein endothelial cells (HUVECs), respectively. Also, miR-130b-3p was transferred by exosomes to HUVECs and then promoted angiogenesis and inhibit the expression of PTEN. Furthermore, exosomal miR-130b-3p derived from OSCC cells promoted tumor growth and blood vessel formation in the xenograft mice model. Taken together, we demonstrated that exosome-mediated miR-130b-3p promoted progression and tubular formation in OSCC in vitro and in vivo. These results would provide new insight into exploring biomarkers and effective therapeutic strategies for OSCC.
Keywords: Pten; exosome; miR-130b-3p; oral squamous cell carcinoma; progression; tubular formation.
Copyright © 2021 Yan, Wang, Chen, Guo, Li and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Role of exosomes in the communication and treatment between OSCC and normal cells.Heliyon. 2024 Mar 20;10(7):e28148. doi: 10.1016/j.heliyon.2024.e28148. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38560136 Free PMC article. Review.
-
Human placenta mesenchymal stem cell-derived exosome shuttling microRNA-130b-3p from gestational diabetes mellitus patients targets ICAM-1 and perturbs human umbilical vein endothelial cell angiogenesis.Acta Diabetol. 2022 Aug;59(8):1091-1107. doi: 10.1007/s00592-022-01910-2. Epub 2022 Jun 8. Acta Diabetol. 2022. PMID: 35676597
-
M2 Macrophages-Derived Exosomal miRNA-23a-3p Promotes the Progression of Oral Squamous Cell Carcinoma by Targeting PTEN.Curr Issues Mol Biol. 2023 Jun 7;45(6):4936-4947. doi: 10.3390/cimb45060314. Curr Issues Mol Biol. 2023. PMID: 37367063 Free PMC article.
-
Exosomal microRNA-125a-3p from human adipose-derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN.Mol Cell Biochem. 2022 Jan;477(1):115-127. doi: 10.1007/s11010-021-04251-w. Epub 2021 Sep 28. Mol Cell Biochem. 2022. PMID: 34581942
-
Comprehension of PTEN-Regulated MicroRNA Profiling in Oral Premalignant Lesions: A Critical Link to Early Detection of Oral Squamous Cell Carcinoma.Cureus. 2025 Apr 16;17(4):e82343. doi: 10.7759/cureus.82343. eCollection 2025 Apr. Cureus. 2025. PMID: 40385769 Free PMC article. Review.
Cited by
-
Emerging role of exosome-derived non-coding RNAs in tumor-associated angiogenesis of tumor microenvironment.Front Mol Biosci. 2023 Aug 4;10:1220193. doi: 10.3389/fmolb.2023.1220193. eCollection 2023. Front Mol Biosci. 2023. PMID: 37602326 Free PMC article. Review.
-
Role of exosomes in the communication and treatment between OSCC and normal cells.Heliyon. 2024 Mar 20;10(7):e28148. doi: 10.1016/j.heliyon.2024.e28148. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38560136 Free PMC article. Review.
-
Non-coding RNAs in ferroptotic cancer cell death pathway: meet the new masters.Hum Cell. 2022 Jul;35(4):972-994. doi: 10.1007/s13577-022-00699-0. Epub 2022 Apr 12. Hum Cell. 2022. PMID: 35415781 Review.
-
Emerging functions and clinical applications of exosomal microRNAs in diseases.Noncoding RNA Res. 2023 May 9;8(3):350-362. doi: 10.1016/j.ncrna.2023.05.004. eCollection 2023 Sep. Noncoding RNA Res. 2023. PMID: 37250456 Free PMC article. Review.
-
STAT3/miR-130b-3p/MBNL1 feedback loop regulated by mTORC1 signaling promotes angiogenesis and tumor growth.J Exp Clin Cancer Res. 2022 Oct 11;41(1):297. doi: 10.1186/s13046-022-02513-z. J Exp Clin Cancer Res. 2022. PMID: 36217202 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

