Biocompatibility and Viscoelastic Properties of Injectable Resilin-Like Polypeptide and Hyaluronan Hybrid Hydrogels in Rabbit Vocal Folds
- PMID: 33829078
- PMCID: PMC8022864
- DOI: 10.1007/s40883-019-00094-6
Biocompatibility and Viscoelastic Properties of Injectable Resilin-Like Polypeptide and Hyaluronan Hybrid Hydrogels in Rabbit Vocal Folds
Abstract
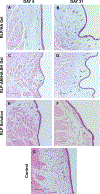
Vocal fold scar, characterized by alterations in the lamina propria extracellular matrix, disrupts normal voice quality and function. Due to a lack of satisfactory clinical treatments, there is a need for tissue engineering strategies to restore voice. Candidate biomaterials for vocal fold tissue engineering must match the unique biomechanical and viscoelastic properties of native tissue without provoking inflammation. We sought to introduce elastomeric properties to hyaluronic acid (HA)-based biomaterials by incorporating resilin-like polypeptide (RLP) into hybrid hydrogels. Physically crosslinked RLP/HA and chemically crosslinked RLP-acrylamide/thiolated HA (RLP-AM/HA-SH) hydrogels were fabricated using cytocompatible chemistries. Mechanical properties of hydrogels were assessed in vitro using oscillatory rheology. Hybrid hydrogels were injected into rabbit vocal folds and tissues were assessed using rheology and histology. A small number of animals underwent acute vocal fold injury followed by injection of RLP-AM/HA-SH hydrogel alone or as a carrier for human bone marrow mesenchymal stem cells (BM-MSCs). Rheological testing confirmed that mechanical properties of materials in vitro resembled native vocal fold tissue and that viscoelasticity of vocal fold mucosa was preserved days 5 and 21 after injection. Histological analysis revealed that hybrid hydrogels provoked only mild inflammation in vocal fold lamina propria with demonstrated safety in the airway for up to 3 weeks, confirming acute biocompatibility of crosslinking chemistries. After acute injury, RLP-AM/HA-SH gel with and without BM-MSCs did not result in adverse effects or increased inflammation. Collectively, results indicate that RLP and HA-based hybrid hydrogels are highly promising for engineering the vocal fold lamina propria.
Keywords: Biocompatibility; Injectable hydrogels; Resilin; Viscoelastic properties; Vocal folds.
Conflict of interest statement
Conflict of Interest The authors declare that they have no conflict of interest.
Figures




References
-
- Hirano M Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr. 1974;26:89–94. - PubMed
-
- Hirano M, Kurita S, Nakashima T. The structure of the vocal folds. In: Stevens KN, Hirano M, editors. Vocal fold physiology. Tokyo: University of Tokyo Press; 1981. p. 33–43.
-
- Gray SD. Cellular physiology of the vocal folds. Otolaryngol Clin N Am. 2000;33:679–98. - PubMed
-
- Gray SD, Alipour F, Titze IR, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. - PubMed
-
- Woo P, Casper J, Colton R, Brewer D. Diagnosis and treatment of persistent dysphonia after laryngeal surgery: a retrospective analysis of 62 patients. Laryngoscope. 1994;104:1084–91. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
