Mitochondrial-targeted methionine sulfoxide reductase overexpression increases the production of oxidative stress in mitochondria from skeletal muscle
- PMID: 33829213
- PMCID: PMC8023689
- DOI: 10.31491/apt.2020.03.012
Mitochondrial-targeted methionine sulfoxide reductase overexpression increases the production of oxidative stress in mitochondria from skeletal muscle
Abstract
Objective: Mitochondrial dysfunction comprises part of the etiology of myriad health issues, particularly those that occur with advancing age. Methionine sulfoxide reductase A (MsrA) is a ubiquitous protein oxidation repair enzyme that specifically and catalytically reduces a specific epimer of oxidized methionine: methionine sulfoxide. In this study, we tested the ways in which mitochondrial bioenergetic functions are affected by increasing MsrA expression in different cellular compartments.
Methods: In this study, we tested the function of isolated mitochondria, including free radical generation, ATP production, and respiration, from the skeletal muscle of two lines of transgenic mice with increased MsrA expression: mitochondria-targeted MsrA overexpression or cytosol-targeted MsrA overexpression.
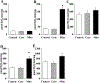
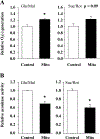
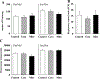
Results: Surprisingly, in the samples from mice with mitochondrial-targeted MsrA overexpression, we found dramatically increased free radical production though no specific defect in respiration, ATP production, or membrane potential. Among the electron transport chain complexes, we found the activity of complex I was specifically reduced in mitochondrial MsrA transgenic mice. In mice with cytosolic-targeted MsrA overexpression, we found no significant alteration made to any of these parameters of mitochondrial energetics.
Conclusions: There is also a growing amount of evidence that MsrA is a functional requirement for sustaining optimal mitochondrial respiration and free radical generation. MsrA is also known to play a partial role in maintaining normal protein homeostasis by specifically repairing oxidized proteins. Our studies highlight a potential novel role for MsrA in regulating the activity of mitochondrial function through its interaction with the mitochondrial proteome.
Keywords: Superoxide; electron transport chain; mitochondria; oxidative stress; protein homeostasis.
Figures

References
-
- Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nature reviews Molecular cell biology, 2010, 11(9): 655–667. - PubMed
-
- Moosmann B. Respiratory chain cysteine and methionine usage indicate a causal role for thiyl radicals in aging. Experimental Gerontology, 2011, 46(2–3):164–169. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
