Systemic administration of a β2-adrenergic receptor agonist reduces mechanical allodynia and suppresses the immune response to surgery in a rat model of persistent post-incisional hypersensitivity
- PMID: 33829907
- PMCID: PMC8040570
- DOI: 10.1177/1744806921997206
Systemic administration of a β2-adrenergic receptor agonist reduces mechanical allodynia and suppresses the immune response to surgery in a rat model of persistent post-incisional hypersensitivity
Abstract
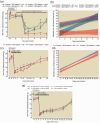
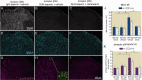
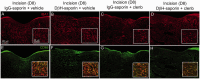
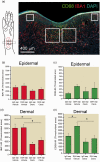
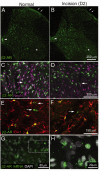
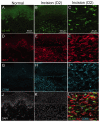
Beta 2 adrenergic receptor (β2 AR) activation in the central and peripheral nervous system has been implicated in nociceptive processing in acute and chronic pain settings with anti-inflammatory and anti-allodynic effects of β2-AR mimetics reported in several pain states. In the current study, we examined the therapeutic efficacy of the β2-AR agonist clenbuterol in a rat model of persistent postsurgical hypersensitivity induced by disruption of descending noradrenergic signaling in rats with plantar incision. We used growth curve modeling of ipsilateral mechanical paw withdrawal thresholds following incision to examine effects of treatment on postoperative trajectories. Depletion of spinal noradrenergic neurons delayed recovery of hypersensitivity following incision evident as a flattened slope compared to non-depleted rats (-1.8 g/day with 95% CI -2.4 to -1.085, p < 0.0001). Chronic administration of clenbuterol reduced mechanical hypersensitivity evident as a greater initial intercept in noradrenergic depleted (6.2 g with 95% CI 1.6 to 10.8, p = 0.013) and non-depleted rats (5.4 g with 95% CI 1.2 to 9.6, p = 0.018) with plantar incision compared to vehicle treated rats. Despite a persistent reduction in mechanical hypersensitivity, clenbuterol did not alter the slope of recovery when modeled over several days (p = 0.053) or five weeks in depleted rats (p = 0.64). Systemic clenbuterol suppressed the enhanced microglial activation in depleted rats and reduced the density of macrophage at the site of incision. Direct spinal infusion of clenbuterol failed to reduce mechanical hypersensitivity in depleted rats with incision suggesting that beneficial effects of β2-AR stimulation in this model are largely peripherally mediated. Lastly, we examined β2-AR distribution in the spinal cord and skin using in-situ hybridization and IHC. These data add to our understanding of the role of β2-ARs in the nervous system on hypersensitivity after surgical incision and extend previously observed anti-inflammatory actions of β2-AR agonists to models of surgical injury.
Keywords: Postoperative pain; acute to chronic pain transition; glial plasticity; growth curve modeling; surgery.
Conflict of interest statement
Figures
Similar articles
-
Disruption of Spinal Noradrenergic Activation Delays Recovery of Acute Incision-Induced Hypersensitivity and Increases Spinal Glial Activation in the Rat.J Pain. 2016 Feb;17(2):190-202. doi: 10.1016/j.jpain.2015.10.009. Epub 2015 Nov 3. J Pain. 2016. PMID: 26545342 Free PMC article.
-
Mechanical pain hypersensitivity after incisional surgery is enhanced in rats subjected to neonatal peripheral inflammation: effects of N-methyl-D-aspartate receptor antagonists.Anesthesiology. 2007 Jun;106(6):1204-12. doi: 10.1097/01.anes.0000267604.40258.d1. Anesthesiology. 2007. PMID: 17525596
-
β2-Adrenergic Receptor Agonist Clenbuterol Protects Against Acute Ischemia/Reperfusion-Induced Arrhythmia by Regulation of Akt/eNOS/NO/Cx43 Signaling Pathway.Pharmacol Res Perspect. 2025 Feb;13(1):e70070. doi: 10.1002/prp2.70070. Pharmacol Res Perspect. 2025. PMID: 39873977 Free PMC article.
-
Does the beta2-agonist clenbuterol help to maintain myocardial potential to recover during mechanical unloading?Circulation. 2005 Aug 30;112(9 Suppl):I51-6. doi: 10.1161/CIRCULATIONAHA.104.525097. Circulation. 2005. PMID: 16159865
-
The β-adrenergic hypothesis of synaptic and microglial impairment in Alzheimer's disease.J Neurochem. 2023 May;165(3):289-302. doi: 10.1111/jnc.15782. Epub 2023 Feb 28. J Neurochem. 2023. PMID: 36799441 Review.
Cited by
-
Widespread hyperexcitability of nociceptor somata outlasts enhanced avoidance behavior after incision injury.Pain. 2025 May 1;166(5):1088-1104. doi: 10.1097/j.pain.0000000000003443. Epub 2024 Oct 22. Pain. 2025. PMID: 39432803
-
Activation of β2-Adrenergic Receptors in Microglia Alleviates Neuropathic Hypersensitivity in Mice.Cells. 2023 Jan 11;12(2):284. doi: 10.3390/cells12020284. Cells. 2023. PMID: 36672219 Free PMC article.
-
Compromised trigemino-coerulean coupling in migraine sensitization can be prevented by blocking beta-receptors in the locus coeruleus.J Headache Pain. 2023 Dec 8;24(1):165. doi: 10.1186/s10194-023-01691-1. J Headache Pain. 2023. PMID: 38062355 Free PMC article.
-
Widespread latent hyperactivity of nociceptors outlasts enhanced avoidance behavior following incision injury.bioRxiv [Preprint]. 2024 Jan 31:2024.01.30.578108. doi: 10.1101/2024.01.30.578108. bioRxiv. 2024. Update in: Pain. 2025 May 1;166(5):1088-1104. doi: 10.1097/j.pain.0000000000003443. PMID: 38352319 Free PMC article. Updated. Preprint.
-
Sympathectomy Ameliorates CFA-Induced Mechanical Allodynia via Modulating Phenotype of Macrophages in Sensory Ganglion in Mice.J Inflamm Res. 2022 Nov 11;15:6263-6274. doi: 10.2147/JIR.S388322. eCollection 2022. J Inflamm Res. 2022. PMID: 36386581 Free PMC article.
References
-
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. - PubMed
-
- De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain 2011; 152: 2701–2709. - PMC - PubMed
-
- Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008; 138: 22–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

