A calcineurin-mediated scaling mechanism that controls a K+-leak channel to regulate morphogen and growth factor transcription
- PMID: 33830014
- PMCID: PMC8110307
- DOI: 10.7554/eLife.60691
A calcineurin-mediated scaling mechanism that controls a K+-leak channel to regulate morphogen and growth factor transcription
Abstract
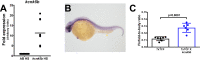
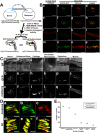
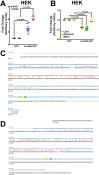
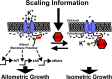
The increase in activity of the two-pore potassium-leak channel Kcnk5b maintains allometric juvenile growth of adult zebrafish appendages. However, it remains unknown how this channel maintains allometric growth and how its bioelectric activity is regulated to scale these anatomical structures. We show the activation of Kcnk5b is sufficient to activate several genes that are part of important development programs. We provide in vivo transplantation evidence that the activation of gene transcription is cell autonomous. We also show that Kcnk5b will induce the expression of different subsets of the tested developmental genes in different cultured mammalian cell lines, which may explain how one electrophysiological stimulus can coordinately regulate the allometric growth of diverse populations of cells in the fin that use different developmental signals. We also provide evidence that the post-translational modification of serine 345 in Kcnk5b by calcineurin regulates channel activity to scale the fin. Thus, we show how an endogenous bioelectric mechanism can be regulated to promote coordinated developmental signaling to generate and scale a vertebrate appendage.
Keywords: calcineurin; developmental biology; electrophysiology; potassium channel; proportional growth; tissue scaling; transcription; zebrafish.
Plain language summary
Organs, limbs, fins and tails are made of multiple tissues whose growth is controlled by specific signals and genetic programmes. All these different cell populations must work together during development or regeneration to form a complete structure that is the right size in relation to the rest of the body. Growing evidence suggests that this synchronicity might be down to electric signals, which are created by movements of charged particles in and out of cells. In particular, previous work has identified two factors that control the development of fins in fish: the Kcnk5b potassium-leak channel, which allows positive ions to cross the cell membrane; and an enzyme called calcineurin, which can modify the activity of proteins. Kcnk5b and calcineurin seem to play similar roles in the proportional growth of the fins in relation to the body, but exactly how was unknown. To investigate this question, Yi et al. used genetically modified zebrafish to show how the Kcnk5b channel could control genes responsible for appendage growth. However, their tests on different cell types revealed that potassium movement through the Kcnk5b channel leads to different sets of developmental genes being turned on, depending on the tissue type of the cell. This could explain how one type of signal (in this case, movement of ions) can coordinate the growth of a wide range of tissues that use different combinations of developmental genes to form. Kcnk5b therefore appears to coordinate the regulation of the various combinations of genes needed for different fin tissues to develop, so that every component grows in a proportional, synchronized manner. Yi et al. also showed that calcineurin can modify the Kcnk5b channel to control its activity. In turn, this affects the movement of potassium ions across the membrane, changing electrical activity and, as a consequence, the proportional growth of the fin. Further work should explore how Kcnk5b and calcineurin link to other signals that regulate the size of fins and limbs. Ultimately, a finer understanding of the molecules controlling the growth of body parts will be useful in fields such as regenerative medicine or stem cell biology, which attempt to build organs for clinical therapies.
© 2021, Yi et al.
Conflict of interest statement
CY, TS, EA, SW, TX, SC, XY, KG, MW, AE, CA No competing interests declared
Figures










Similar articles
-
Bioelectric-calcineurin signaling module regulates allometric growth and size of the zebrafish fin.Sci Rep. 2018 Jul 10;8(1):10391. doi: 10.1038/s41598-018-28450-6. Sci Rep. 2018. PMID: 29991812 Free PMC article.
-
The scale of zebrafish pectoral fin buds is determined by intercellular K+ levels and consequent Ca2+-mediated signaling via retinoic acid regulation of Rcan2 and Kcnk5b.PLoS Biol. 2024 Mar 25;22(3):e3002565. doi: 10.1371/journal.pbio.3002565. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38527087 Free PMC article.
-
Bioelectric signaling regulates size in zebrafish fins.PLoS Genet. 2014 Jan;10(1):e1004080. doi: 10.1371/journal.pgen.1004080. Epub 2014 Jan 16. PLoS Genet. 2014. PMID: 24453984 Free PMC article.
-
Signaling networks organizing regenerative growth of the zebrafish fin.Trends Genet. 2015 Jun;31(6):336-43. doi: 10.1016/j.tig.2015.03.012. Epub 2015 Apr 27. Trends Genet. 2015. PMID: 25929514 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Hallmarks of regeneration.Cell Stem Cell. 2024 Sep 5;31(9):1244-1261. doi: 10.1016/j.stem.2024.07.007. Epub 2024 Aug 19. Cell Stem Cell. 2024. PMID: 39163854 Review.
-
Cellular signaling pathways as plastic, proto-cognitive systems: Implications for biomedicine.Patterns (N Y). 2023 Apr 26;4(5):100737. doi: 10.1016/j.patter.2023.100737. eCollection 2023 May 12. Patterns (N Y). 2023. PMID: 37223267 Free PMC article. Review.
-
Growth patterns of caudal fin rays are informed by both external signals from the regenerating organ and remembered identity autonomous to the local tissue.bioRxiv [Preprint]. 2024 Jul 11:2024.03.29.586899. doi: 10.1101/2024.03.29.586899. bioRxiv. 2024. Update in: Dev Biol. 2024 Nov;515:121-128. doi: 10.1016/j.ydbio.2024.07.008. PMID: 38585773 Free PMC article. Updated. Preprint.
-
Growth patterns of caudal fin rays are informed by both external signals from the regenerating organ and remembered identity autonomous to the local tissue.Dev Biol. 2024 Nov;515:121-128. doi: 10.1016/j.ydbio.2024.07.008. Epub 2024 Jul 18. Dev Biol. 2024. PMID: 39029570
-
KCNK18 Biallelic Variants Associated with Intellectual Disability and Neurodevelopmental Disorders Alter TRESK Channel Activity.Int J Mol Sci. 2021 Jun 4;22(11):6064. doi: 10.3390/ijms22116064. Int J Mol Sci. 2021. PMID: 34199759 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

