Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection
- PMID: 33830018
- PMCID: PMC8064747
- DOI: 10.7554/eLife.68808
Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection
Abstract
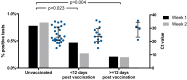
The BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) is being utilised internationally for mass COVID-19 vaccination. Evidence of single-dose protection against symptomatic disease has encouraged some countries to opt for delayed booster doses of BNT162b2, but the effect of this strategy on rates of asymptomatic SARS-CoV-2 infection remains unknown. We previously demonstrated frequent pauci- and asymptomatic SARS-CoV-2 infection amongst healthcare workers (HCWs) during the UK's first wave of the COVID-19 pandemic, using a comprehensive PCR-based HCW screening programme (Rivett et al., 2020; Jones et al., 2020). Here, we evaluate the effect of first-dose BNT162b2 vaccination on test positivity rates and find a fourfold reduction in asymptomatic infection amongst HCWs ≥12 days post-vaccination. These data provide real-world evidence of short-term protection against asymptomatic SARS-CoV-2 infection following a single dose of BNT162b2 vaccine, suggesting that mass first-dose vaccination will reduce SARS-CoV-2 transmission, as well as the burden of COVID-19 disease.
Keywords: BNT162b2; COVID-19; Pfizer-BioNTech; SARS-CoV-2; asymptomatic; epidemiology; global health; human; infectious disease; microbiology; vaccination.
© 2021, Jones et al.
Conflict of interest statement
NJ, LR, SS, RS, BW, CW, MF, JW, NQ, AS, IG, PL, GW, NM, MW No competing interests declared, RH Dr Howes was employed by AstraZeneca PLC during the period of study and preparation of this manuscript.
Figures
References
-
- Department of Health and Social Care Optimising the COVID-19 vaccination programme for maximum short-term impact. [February 15, 2020];2021 https://www.gov.uk/government/publications/prioritising-the-first-covid-...
-
- Jones NK, Rivett L, Sparkes D, Forrest S, Sridhar S, Young J, Pereira-Dias J, Cormie C, Gill H, Reynolds N, Wantoch M, Routledge M, Warne B, Levy J, Córdova Jiménez WD, Samad FNB, McNicholas C, Ferris M, Gray J, Gill M, Curran MD, Fuller S, Chaudhry A, Shaw A, Bradley JR, Hannon GJ, Goodfellow IG, Dougan G, Smith KG, Lehner PJ, Wright G, Matheson NJ, Baker S, Weekes MP, CITIID-NIHR COVID-19 BioResource Collaboration Effective control of SARS-CoV-2 transmission between healthcare workers during a period of diminished community prevalence of COVID-19. eLife. 2020;9:e59391. doi: 10.7554/eLife.59391. - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Rivett L, Sridhar S, Sparkes D, Routledge M, Jones NK, Forrest S, Young J, Pereira-Dias J, Hamilton WL, Ferris M, Torok ME, Meredith L, Curran MD, Fuller S, Chaudhry A, Shaw A, Samworth RJ, Bradley JR, Dougan G, Smith KG, Lehner PJ, Matheson NJ, Wright G, Goodfellow IG, Baker S, Weekes MP, CITIID-NIHR COVID-19 BioResource Collaboration Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife. 2020;9:e58728. doi: 10.7554/eLife.58728. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

