Inflammation rapidly recruits mammalian GMP and MDP from bone marrow into regional lymphatics
- PMID: 33830019
- PMCID: PMC8137144
- DOI: 10.7554/eLife.66190
Inflammation rapidly recruits mammalian GMP and MDP from bone marrow into regional lymphatics
Abstract
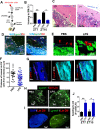
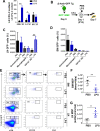
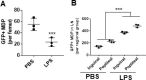
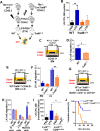
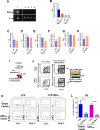
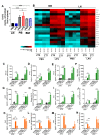
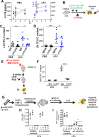
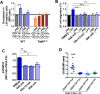
Innate immune cellular effectors are actively consumed during systemic inflammation, but the systemic traffic and the mechanisms that support their replenishment remain unknown. Here, we demonstrate that acute systemic inflammation induces the emergent activation of a previously unrecognized system of rapid migration of granulocyte-macrophage progenitors and committed macrophage-dendritic progenitors, but not other progenitors or stem cells, from bone marrow (BM) to regional lymphatic capillaries. The progenitor traffic to the systemic lymphatic circulation is mediated by Ccl19/Ccr7 and is NF-κB independent, Traf6/IκB-kinase/SNAP23 activation dependent, and is responsible for the secretion of pre-stored Ccl19 by a subpopulation of CD205+/CD172a+ conventional dendritic cells type 2 and upregulation of BM myeloid progenitor Ccr7 signaling. Mature myeloid Traf6 signaling is anti-inflammatory and necessary for lymph node myeloid cell development. This report unveils the existence and the mechanistic basis of a very early direct traffic of myeloid progenitors from BM to lymphatics during inflammation.
Keywords: bone marrow; circulation; endotoxemia; human; immunology; inflammation; lymphatics; medicine; mouse; myeloid progenitors.
Plain language summary
When the body becomes infected with disease-causing pathogens, such as bacteria, the immune system activates various mechanisms which help to fight off the infection. One of the immune system’s first lines of defense is to launch an inflammatory response that helps remove the pathogen and recruit other immune cells. However, this response can become overactivated, leading to severe inflammatory conditions that damage healthy cells and tissues. A second group of cells counteract this over inflammation and are different to the ones involved in the early inflammatory response. Both types of cells – inflammatory and anti-inflammatory – develop from committed progenitors, which, unlike stem cells, are already destined to become a certain type of cell. These committed progenitors reside in the bone marrow and then rapidly travel to secondary lymphoid organs, such as the lymph nodes, where they mature into functioning immune cells. During this journey, committed progenitors pass from the bone marrow to the lymphatic vessels that connect up the different secondary lymphoid organs, and then spread to all tissues in the body. Yet, it is not fully understood what exact route these cells take and what guides them towards these lymphatic tissues during inflammation. To investigate this, Serrano-Lopez, Hegde et al. used a combination of techniques to examine the migration of progenitor cells in mice that had been treated with lethal doses of a bacterial product that triggers inflammation. This revealed that as early as one to three hours after the onset of infection, progenitor cells were already starting to travel from the bone marrow towards lymphatic vessels. Serrano-Lopez, Hegde et al. found that a chemical released by an “alarm” immune cell already residing in secondary lymphoid organs attracted these progenitor cells towards the lymphatic tissue. Further experiments showed that the progenitor cells travelling to secondary lymphoid organs were already activated by bacterial products. They then follow the chemical released by alarm immune cells ready to respond to the immune challenge and suppress inflammation. These committed progenitors were also found in the inflamed lymph nodes of patients. These findings suggest this rapid circulation of progenitors is a mechanism of defense that contributes to the fight against severe inflammation. Altering how these cells migrate from the bone marrow to secondary lymphoid organs could provide a more effective treatment for inflammatory conditions and severe infections. However, these approaches would need to be tested further in the laboratory and in clinical trials.
Conflict of interest statement
JS, SH, SK, JS, JF, AW, PR, YR, LC, HG, HG, SL, IM, JS, DS, JC No competing interests declared
Figures






Similar articles
-
Anatomy, Lymphatic System.2023 Mar 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30020619 Free Books & Documents.
-
Lead Transiently Promotes Granulocyte-Macrophage Progenitor Differentiation and Subsequently Suppresses Common Myeloid Progenitor Differentiation.Toxicol Sci. 2017 Dec 1;160(2):268-283. doi: 10.1093/toxsci/kfx176. Toxicol Sci. 2017. PMID: 28973681
-
Diabetes induces myeloid bias in bone marrow progenitors associated with enhanced wound macrophage accumulation and impaired healing.J Pathol. 2019 Dec;249(4):435-446. doi: 10.1002/path.5330. Epub 2019 Aug 28. J Pathol. 2019. PMID: 31342513 Free PMC article.
-
NK Cell Precursors in Human Bone Marrow in Health and Inflammation.Front Immunol. 2019 Aug 28;10:2045. doi: 10.3389/fimmu.2019.02045. eCollection 2019. Front Immunol. 2019. PMID: 31555276 Free PMC article. Review.
-
Neutrophil Interactions with the Lymphatic System.Cells. 2021 Aug 17;10(8):2106. doi: 10.3390/cells10082106. Cells. 2021. PMID: 34440875 Free PMC article. Review.
Cited by
-
Behind the monocyte's mystique: uncovering their developmental trajectories and fates.Discov Immunol. 2023 Jul 19;2(1):kyad008. doi: 10.1093/discim/kyad008. eCollection 2023. Discov Immunol. 2023. PMID: 38567063 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

