New gene discoveries in skeletal diseases with short stature
- PMID: 33830070
- PMCID: PMC8183621
- DOI: 10.1530/EC-21-0083
New gene discoveries in skeletal diseases with short stature
Abstract
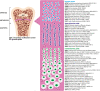
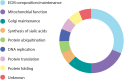
In the last decade, the widespread use of massively parallel sequencing has considerably boosted the number of novel gene discoveries in monogenic skeletal diseases with short stature. Defects in genes playing a role in the maintenance and function of the growth plate, the site of longitudinal bone growth, are a well-known cause of skeletal diseases with short stature. However, several genes involved in extracellular matrix composition or maintenance as well as genes partaking in various biological processes have also been characterized. This review aims to describe the latest genetic findings in spondyloepiphyseal dysplasias, spondyloepimetaphyseal dysplasias, and some monogenic forms of isolated short stature. Some examples of novel genetic mechanisms leading to skeletal conditions with short stature will be described. Strategies on how to successfully characterize novel skeletal phenotypes with short stature and genetic approaches to detect and validate novel gene-disease correlations will be discussed in detail. In summary, we review the latest gene discoveries underlying skeletal diseases with short stature and emphasize the importance of characterizing novel molecular mechanisms for genetic counseling, for an optimal management of the disease, and for therapeutic innovations.
Figures

References
-
- Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, Nishimura G, Robertson S, Sangiorgi L, Savarirayan R. et al. Nosology and classification of genetic skeletal disorders: 2019 revision. American Journal of Medical Genetics. Part A 2019. 179 2393–2419. ( 10.1002/ajmg.a.61366) - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

