Hypoxia and viral infectious diseases
- PMID: 33830079
- PMCID: PMC8119216
- DOI: 10.1172/jci.insight.147190
Hypoxia and viral infectious diseases
Abstract
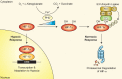
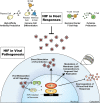
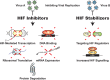
Oxygen-sensing mechanisms allow cells to adapt and respond to changes in cellular oxygen tension, including hypoxic conditions. Hypoxia-inducible factor (HIF) is a central mediator in this fundamental adaptive response, and has critical functions in normal and disease physiology. Viruses have been shown to manipulate HIFs during their life cycle to facilitate replication and invasion. Conversely, HIFs are also implicated in the development of the host immune system and response to viral infections. Here, we highlight the recent revelations of host-pathogen interactions that involve the hypoxic response pathway and the role of HIF in emerging viral infectious diseases, as well as discussing potential antiviral therapeutic strategies targeting the HIF signaling axis.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

