Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata
- PMID: 33830087
- PMCID: PMC8119218
- DOI: 10.1172/jci.insight.142205
Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata
Abstract
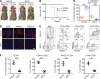
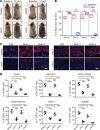
The Janus kinase/signal transducers and activators of transcription (JAK/STAT) are key intracellular mediators in the signal transduction of many cytokines and growth factors. Common γ chain cytokines and interferon-γ that use the JAK/STAT pathway to induce biological responses have been implicated in the pathogenesis of alopecia areata (AA), a T cell-mediated autoimmune disease of the hair follicle. We previously showed that therapeutic targeting of JAK/STAT pathways using the first-generation JAK1/2 inhibitor, ruxolitinib, and the pan-JAK inhibitor, tofacitinib, was highly effective in the treatment of human AA, as well as prevention and reversal of AA in the C3H/HeJ mouse model. To better define the role of individual JAKs in the pathogenesis of AA, in this study, we tested and compared the efficacy of several next-generation JAK-selective inhibitors in the C3H/HeJ mouse model of AA, using both systemic and topical delivery. We found that JAK1-selective inhibitors as well as JAK3-selective inhibitors robustly induced hair regrowth and decreased AA-associated inflammation, whereas several JAK2-selective inhibitors failed to restore hair growth in treated C3H/HeJ mice with AA. Unlike JAK1, which is broadly expressed in many tissues, JAK3 expression is largely restricted to hematopoietic cells. Our study demonstrates inhibiting JAK3 signaling is sufficient to prevent and reverse disease in the preclinical model of AA.
Keywords: Autoimmunity; Inflammation; Skin; T cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

