Assessing treatment response after intravesical bacillus Calmette-Guerin induction cycle: are routine bladder biopsies necessary?
- PMID: 33830306
- PMCID: PMC8519823
- DOI: 10.1007/s00345-021-03690-w
Assessing treatment response after intravesical bacillus Calmette-Guerin induction cycle: are routine bladder biopsies necessary?
Abstract
Purpose: To determine the need for routine bladder biopsies (BBs) in assessing response to the induction cycle of intravesical bacillus Calmette-Guérin (BCG) for high-risk non-muscle-invasive bladder cancer (NMIBC).
Methods: Our prospectively maintained NMIBC database was queried to identify patients with high-risk disease (carcinoma in situ, high-grade Ta/T1) who underwent BBs after BCG induction cycle. Urine cytology, cystoscopy, and BBs findings were evaluated.
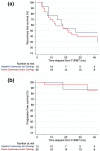
Results: A total of 219 patients met the inclusion criteria. Urine cytology was positive in 20 patients and negative in 199; cystoscopy was positive in 35 patients, suspicious in 32 and normal in 152 patients. BBs yielded bladder cancer (BCa) in 43 (19.6%) patients, with a BCa rate of 9.3% in patients with negative cytology and cystoscopy as opposed to 38.0% in patients whereby one or both exams were suspicious/positive. The diagnostic accuracy of urine cytology, cystoscopy, and combined tests was 0.56, 0.70, and 0.71, respectively. The negative predictive value of combined tests was 90.7%. Performing BBs only in patients with positive cytology and/or positive/suspicious cystoscopy would have spared 140 (64%) patients to undergo this procedure while missing BCa in 13 (9.3%) of them, representing 30% of all BCa cases.
Conclusion: Performing BBs only in patients with positive cytology and suspicious/positive cystoscopy would spare 64% of un-necessary BBs but miss a non-negligible number of BCas. While no data are available regarding the potential consequences of missing such BCas, such information should be taken into account in patient's counselling.
Keywords: BCG response; Bladder biopsy; High-risk Bladder Cancer; NMIBC treatment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare they have no potential conflicts of interest to disclose.
Figures
Comment in
-
Can artificial intelligence help reduce unnecessary bladder biopsies? Comment on "Assessing treatment response after intravesical bacillus Calmette-Guerin induction cycle: are routine bladder biopsies necessary".World J Urol. 2022 May;40(5):1241-1242. doi: 10.1007/s00345-021-03748-9. Epub 2021 Jun 8. World J Urol. 2022. PMID: 34101020 No abstract available.
Similar articles
-
Assessing treatment response after induction Bacillus Calmette-Guerin for carcinoma in situ of the urinary bladder: can post-induction random bladder biopsies be avoided?Cytopathology. 2014 Apr;25(2):108-11. doi: 10.1111/cyt.12064. Epub 2013 Apr 2. Cytopathology. 2014. PMID: 23551700
-
Phase I trial of intravesical Bacillus Calmette-Guérin combined with intravenous pembrolizumab in recurrent or persistent high-grade non-muscle-invasive bladder cancer after previous Bacillus Calmette-Guérin treatment.World J Urol. 2021 Oct;39(10):3807-3813. doi: 10.1007/s00345-021-03716-3. Epub 2021 May 8. World J Urol. 2021. PMID: 33966128 Clinical Trial.
-
The value of transurethral bladder biopsy after intravesical bacillus Calmette-Guérin instillation therapy for nonmuscle invasive bladder cancer: a retrospective, single center study and cumulative analysis of the literature.J Urol. 2012 Sep;188(3):748-53. doi: 10.1016/j.juro.2012.05.015. Epub 2012 Jul 20. J Urol. 2012. PMID: 22819422 Review.
-
Is transurethral biopsy of the bladder necessary after 3 months to evaluate response to bacillus Calmette-Guerin therapy?J Urol. 1999 Sep;162(3 Pt 1):708-9. doi: 10.1097/00005392-199909010-00020. J Urol. 1999. PMID: 10458348 Clinical Trial.
-
ICUD-SIU International Consultation on Bladder Cancer 2017: management of non-muscle invasive bladder cancer.World J Urol. 2019 Jan;37(1):51-60. doi: 10.1007/s00345-018-2438-9. Epub 2018 Aug 14. World J Urol. 2019. PMID: 30109483 Review.
Cited by
-
Can artificial intelligence help reduce unnecessary bladder biopsies? Comment on "Assessing treatment response after intravesical bacillus Calmette-Guerin induction cycle: are routine bladder biopsies necessary".World J Urol. 2022 May;40(5):1241-1242. doi: 10.1007/s00345-021-03748-9. Epub 2021 Jun 8. World J Urol. 2022. PMID: 34101020 No abstract available.
-
Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 1: General Issues and Marker Expression.Int J Mol Sci. 2022 Jul 15;23(14):7819. doi: 10.3390/ijms23147819. Int J Mol Sci. 2022. PMID: 35887164 Free PMC article. Review.
-
Oncological outcomes and prognostic implications of T1 histo-anatomic substaging in the management of high-Grade non-muscle invasive bladder cancer: results from a large single centre series.World J Urol. 2024 Dec 26;43(1):47. doi: 10.1007/s00345-024-05410-6. World J Urol. 2024. PMID: 39724414
-
Conservative treatment for high-risk NMIBC failing BCG treatment: who benefits from adding electromotive drug administration (EMDA) of mitomycin C (MMC) to a second BCG induction cycle?World J Urol. 2023 May;41(5):1329-1335. doi: 10.1007/s00345-023-04372-5. Epub 2023 Mar 27. World J Urol. 2023. PMID: 36971825 Free PMC article.
-
Latest Developments and Current Problems in Bladder Cancer.World J Urol. 2021 Nov;39(11):4009-4010. doi: 10.1007/s00345-021-03857-5. World J Urol. 2021. PMID: 34643773 No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; in press. - PubMed
-
- Ferlay J, Randi G, Bosetti C, et al. Declining mortality from bladder cancer in Europe. BJU Int. 2008;101:11–19. - PubMed
-
- Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma in situ)—2019 update. EurUrol. 2019;76(5):639–657. - PubMed
-
- Murakami T, Ebara S, Saika T, et al. Routine transurethral biopsy of the bladder is not necessary to evaluate the response to bacillus Calmette-Guerin therapy. Acta Med Okayama. 2007;61:341. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous