Pharmacokinetics and Drug-Drug Interactions of Long-Acting Intramuscular Cabotegravir and Rilpivirine
- PMID: 33830459
- PMCID: PMC8249281
- DOI: 10.1007/s40262-021-01005-1
Pharmacokinetics and Drug-Drug Interactions of Long-Acting Intramuscular Cabotegravir and Rilpivirine
Abstract
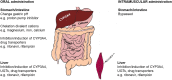
Combined antiretroviral treatments have significantly improved the morbidity and mortality related to HIV infection, thus transforming HIV infection into a chronic disease; however, the efficacy of antiretroviral treatments is highly dependent on the ability of infected individuals to adhere to life-long drug combination therapies. A major milestone in HIV treatment is the marketing of the long-acting intramuscular antiretroviral drugs cabotegravir and rilpivirine, allowing for infrequent drug administration, with the potential to improve adherence to therapy and treatment satisfaction. Intramuscular administration of cabotegravir and rilpivirine leads to differences in pharmacokinetics and drug-drug interaction (DDI) profiles compared with oral administration. A notable difference is the long elimination half-life with intramuscular administration, which reaches 5.6-11.5 weeks for cabotegravir and 13-28 weeks for rilpivirine, compared with 41 and 45 h, respectively, with their oral administration. Cabotegravir and rilpivirine have a low potential to cause DDIs, however these drugs can be victims of DDIs. Cabotegravir is mainly metabolized by UGT1A1, and rilpivirine is mainly metabolized by CYP3A4, therefore these agents are susceptible to DDIs with inhibitors, and particularly inducers of drug-metabolizing enzymes. Intramuscular administration of cabotegravir and rilpivirine has the advantage of eliminating DDIs occurring at the gastrointestinal level, however interactions can still occur at the hepatic level. This review provides insight on the intramuscular administration of drugs and summarizes the pharmacology of long-acting cabotegravir and rilpivirine. Particular emphasis is placed on DDI profiles after oral and intramuscular administration of these antiretroviral drugs.
Conflict of interest statement
Daryl Hodge and Sara Gibbons have no conflicts of interest to declare. David Back and Saye Khoo have received educational grants for the Liverpool drug interaction website (
Figures



References
-
- Cahn P, Andrade-Villanueva J, Arribas JR, Gatell JM, Lama JR, Norton M, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14(7):572–580. - PubMed
-
- Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

