Genomic characterization of small cell carcinomas of the uterine cervix
- PMID: 33830625
- PMCID: PMC8847983
- DOI: 10.1002/1878-0261.12962
Genomic characterization of small cell carcinomas of the uterine cervix
Abstract
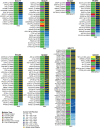
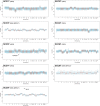
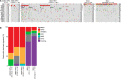
Small cell carcinoma (SCC) of the uterine cervix is a rare and aggressive form of neuroendocrine carcinoma, which resembles small cell lung cancer (SCLC) in its histology and poor survival rate. Here, we sought to define the genetic underpinning of SCCs of the uterine cervix and compare their mutational profiles with those of human papillomavirus (HPV)-positive head and neck squamous cell carcinomas, HPV-positive cervical carcinomas, and SCLCs using publicly available data. Using a combination of whole-exome and targeted massively parallel sequencing, we found that the nine uterine cervix SCCs, which were HPV18-positive (n = 8) or HPV16-positive (n = 1), harbored a low mutation burden, few copy number alterations, and other than TP53 in two cases no recurrently mutated genes. The majority of mutations were likely passenger missense mutations, and only few affected previously described cancer-related genes. Using RNA-sequencing, we identified putative viral integration sites on 18q12.3 and on 8p22 in two SCCs of the uterine cervix. The overall nonsilent mutation rate of uterine cervix SCCs was significantly lower than that of SCLCs, HPV-driven cervical adeno- and squamous cell carcinomas, or HPV-positive head and neck squamous cell carcinomas. Unlike SCLCs, which are reported to harbor almost universal TP53 and RB1 mutations and a dominant tobacco smoke-related signature 4, uterine cervix SCCs rarely harbored mutations affecting these genes (2/9, 22% TP53; 0% RB1) and displayed a dominant aging (67%) or APOBEC mutational signature (17%), akin to HPV-driven cancers, including cervical adeno- and squamous cell carcinomas and head and neck squamous cell carcinomas. Taken together, in contrast to SCLCs, which are characterized by highly recurrent TP53 and RB1 alterations, uterine cervix SCCs were positive for HPV leading to inactivation of the suppressors p53 and RB, suggesting that these SCCs are convergent phenotypes.
Keywords: HPV; mutational signatures; neuroendocrine; small cell carcinoma; uterine cervix; whole-exome sequencing.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
DSK is a founder, consultant, and equity holder of Paige. AI and receives royalties from UpToDate and the American Registry of Pathology, outside of this work. JSR‐F reports receiving personal/consultancy fees from Goldman Sachs, Repare Therapeutics, and Paige.AI, membership of the scientific advisory boards of VolitionRx, Repare Therapeutics, and Paige.AI, membership of the Board of Directors of Grupo Oncoclinicas, and ad hoc membership of the scientific advisory boards of Roche Tissue Diagnostics, Ventana Medical Systems, Novartis, Genentech, and InVicro, outside the scope of this study. BW reports ad hoc membership of the scientific advisory board of Repare Therapeutics, outside the scope of the submitted work. The remaining authors have no conflicts of interest to declare.
Figures

References
-
- Siegel R, Ma J, Zou Z & Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64, 9–29. - PubMed
-
- Wang SS, Sherman ME, Hildesheim A, Lacey JV Jr & Devesa S (2004) Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer 100, 1035–1044. - PubMed
-
- Chen J, Macdonald OK & Gaffney DK (2008) Incidence, mortality, and prognostic factors of small cell carcinoma of the cervix. Obstet Gynecol 111, 1394–1402. - PubMed
-
- Atienza‐Amores M, Guerini‐Rocco E, Soslow RA, Park KJ & Weigelt B (2014) Small cell carcinoma of the gynecologic tract: a multifaceted spectrum of lesions. Gynecol Oncol 134, 410–418. - PubMed
-
- van Meerbeeck JP, Fennell DA & De Ruysscher DK (2011) Small‐cell lung cancer. Lancet 378, 1741–1755. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

