Metal Binding Ability of Small Peptides Containing Cysteine Residues
- PMID: 33830669
- PMCID: PMC8028610
- DOI: 10.1002/open.202000304
Metal Binding Ability of Small Peptides Containing Cysteine Residues
Abstract
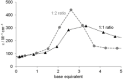
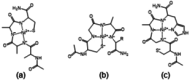
The Cd(II)-, Pb(II)-, Ni(II)- and Zn(II)-complexes of small terminally protected peptides containing CXXX, XXXC, XCCX, CXn C (n=1-3) sequences have been studied with potentiometric, UV/Vis and CD spectroscopic techniques. The cysteine thiolate group is the primary binding site for all studied metal ions, but the presence of a histidyl or aspartyl side chain in the molecule contributes to the stability of the complexes. For two-cysteine containing peptides the (S- ,S- ) coordinated species are formed in the physiological pH range and the stability increases in the Ni(II)<Zn(II)<Pb(II)<Cd(II) order. As a conclusion, the inserting of -CXXC- sequence into the peptide makes the synthesis of peptides with high selectivity to toxic Cd(II) or Pb(II) ion possible. In addition, the spectroscopic characterization of these complexes can contribute to the discovery of the exact binding site and binding mode of longer peptides mimicking the biologically important proteins.
Keywords: cysteine containing peptides; metal complexes; selectivity; spectroscopic measurements; stability constant.
© 2021 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

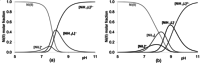
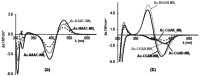




Similar articles
-
Binary and ternary mixed metal complexes of terminally free peptides containing two different histidyl binding sites.J Inorg Biochem. 2013 Nov;128:17-25. doi: 10.1016/j.jinorgbio.2013.07.008. Epub 2013 Jul 16. J Inorg Biochem. 2013. PMID: 23911567
-
Polythiol binding to biologically relevant metal ions.Dalton Trans. 2011 Oct 28;40(40):10434-9. doi: 10.1039/c1dt10562k. Epub 2011 Jul 8. Dalton Trans. 2011. PMID: 21743911
-
Complex formation processes of terminally protected peptides containing two or three histidyl residues. Characterization of the mixed metal complexes of peptides.Dalton Trans. 2008 Oct 7;(37):5059-71. doi: 10.1039/b808323a. Epub 2008 Aug 6. Dalton Trans. 2008. PMID: 18802621
-
Metal Chelation Therapy and Parkinson's Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs.Biomolecules. 2019 Jul 9;9(7):269. doi: 10.3390/biom9070269. Biomolecules. 2019. PMID: 31324037 Free PMC article. Review.
-
Screening, separation and identification of metal-chelating peptides for nutritional, cosmetics and pharmaceutical applications.Food Funct. 2024 Apr 2;15(7):3300-3326. doi: 10.1039/d3fo05765h. Food Funct. 2024. PMID: 38488016 Review.
Cited by
-
CH vs. HC-Promiscuous Metal Sponges in Antimicrobial Peptides and Metallophores.Molecules. 2023 May 9;28(10):3985. doi: 10.3390/molecules28103985. Molecules. 2023. PMID: 37241727 Free PMC article.
-
Histidine Ligated Iron-Sulfur Peptides.Chembiochem. 2022 Jul 19;23(14):e202200202. doi: 10.1002/cbic.202200202. Epub 2022 Jun 23. Chembiochem. 2022. PMID: 35674331 Free PMC article.
-
Cysteine Conjugation: An Approach to Obtain Polymers with Enhanced Muco- and Tissue Adhesion.Int J Mol Sci. 2024 Nov 13;25(22):12177. doi: 10.3390/ijms252212177. Int J Mol Sci. 2024. PMID: 39596243 Free PMC article. Review.
-
Functionalization of bacterial microcompartment shell interior with cysteine containing peptides enhances the iron and cobalt loading capacity.Biometals. 2024 Feb;37(1):267-274. doi: 10.1007/s10534-023-00538-1. Epub 2023 Sep 20. Biometals. 2024. PMID: 37728832
-
Design, Synthesis and Characterization of Mn(II)Cysteine-Tyrosine Dithiocarbamate Complex for against the Cancer on MCF-7 Breast Cancer Cell Line.Asian Pac J Cancer Prev. 2024 Sep 1;25(9):3251-3261. doi: 10.31557/APJCP.2024.25.9.3251. Asian Pac J Cancer Prev. 2024. PMID: 39342604 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

