VHH212 nanobody targeting the hypoxia-inducible factor 1α suppresses angiogenesis and potentiates gemcitabine therapy in pancreatic cancer in vivo
- PMID: 33830713
- PMCID: PMC8330535
- DOI: 10.20892/j.issn.2095-3941.2020.0568
VHH212 nanobody targeting the hypoxia-inducible factor 1α suppresses angiogenesis and potentiates gemcitabine therapy in pancreatic cancer in vivo
Abstract
Objective: We aimed to develop a novel anti-HIF-1α intrabody to decrease gemcitabine resistance in pancreatic cancer patients.
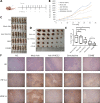
Methods: Surface plasmon resonance and glutathione S-transferase pull-down assays were conducted to identify the binding affinity and specificity of anti-HIF-1α VHH212 [a single-domain antibody (nanobody)]. Molecular dynamics simulation was used to determine the protein-protein interactions between hypoxia-inducible factor-1α (HIF-1α) and VHH212. The real-time polymerase chain reaction (PCR) and Western blot analyses were performed to identify the expressions of HIF-1α and VEGF-A in pancreatic ductal adenocarcinoma cell lines. The efficiency of the VHH212 nanobody in inhibiting the HIF-1 signaling pathway was measured using a dual-luciferase reporter assay. Finally, a PANC-1 xenograft model was developed to evaluate the anti-tumor efficiency of combined treatment. Immunohistochemistry analysis was conducted to detect the expressions of HIF-1α and VEGF-A in tumor tissues.
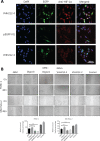
Results: VHH212 was stably expressed in tumor cells with low cytotoxicity, high affinity, specific subcellular localization, and neutralization of HIF-1α in the cytoplasm or nucleus. The binding affinity between VHH212 and the HIF-1α PAS-B domain was 42.7 nM. Intrabody competitive inhibition of the HIF-1α heterodimer with an aryl hydrocarbon receptor nuclear translocator was used to inhibit the HIF-1/VEGF pathway in vitro. Compared with single agent gemcitabine, co-treatment with gemcitabine and a VHH212-encoding adenovirus significantly suppressed tumor growth in the xenograft model with 80.44% tumor inhibition.
Conclusions: We developed an anti-HIF-1α nanobody and showed the function of VHH212 in a preclinical murine model of PANC-1 pancreatic cancer. The combination of VHH212 and gemcitabine significantly inhibited tumor development. These results suggested that combined use of anti-HIF-1α nanobodies with first-line treatment may in the future be an effective treatment for pancreatic cancer.
Keywords: HIF-1α inhibitor; Pancreatic cancer; chemosensitizer; gemcitabine; intracellular antibody; nanobody therapeutic.
Copyright © 2021 Cancer Biology & Medicine.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures



Similar articles
-
[Buyang Huanwu Decoction promotes angiogenesis after oxygen-glucose deprivation/reoxygenation injury of bEnd.3 cells by regulating YAP1/HIF-1α signaling pathway via caveolin-1].Zhongguo Zhong Yao Za Zhi. 2025 Jul;50(14):3847-3856. doi: 10.19540/j.cnki.cjcmm.20250213.705. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40904071 Chinese.
-
Salvianolic Acid B Promotes Placental and Decidual Angiogenesis by Restoring the Normal Expression of Hypoxia-Inducible Factor-1α/Vascular Endothelial Growth Factor in Mice With Recurrent Pregnancy Loss.Am J Reprod Immunol. 2025 Jul;94(1):e70105. doi: 10.1111/aji.70105. Am J Reprod Immunol. 2025. PMID: 40637198
-
Hypoxia-inducible factor 1alpha and vascular endothelial growth factor in Glioblastoma Multiforme: a systematic review going beyond pathologic implications.Oncol Res. 2024 Jul 17;32(8):1239-1256. doi: 10.32604/or.2024.052130. eCollection 2024. Oncol Res. 2024. PMID: 39055895 Free PMC article.
-
MiR-143-5p regulates the proangiogenic potential of human dental pulp stem cells by targeting HIF-1α/RORA under hypoxia: A laboratory investigation in pulp regeneration.Int Endod J. 2024 Dec;57(12):1802-1818. doi: 10.1111/iej.14133. Epub 2024 Aug 10. Int Endod J. 2024. PMID: 39126298
-
Clinicopathological and prognostic significance of hypoxia-inducible factor-1 alpha in lung cancer: a systematic review with meta-analysis.J Huazhong Univ Sci Technolog Med Sci. 2016 Jun;36(3):321-327. doi: 10.1007/s11596-016-1586-7. Epub 2016 Jul 5. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 27376798
Cited by
-
Research Progress and Applications of Multivalent, Multispecific and Modified Nanobodies for Disease Treatment.Front Immunol. 2022 Jan 18;12:838082. doi: 10.3389/fimmu.2021.838082. eCollection 2021. Front Immunol. 2022. PMID: 35116045 Free PMC article.
-
Nanobodies: Robust miniprotein binders in biomedicine.Adv Drug Deliv Rev. 2023 Apr;195:114726. doi: 10.1016/j.addr.2023.114726. Epub 2023 Feb 7. Adv Drug Deliv Rev. 2023. PMID: 36754285 Free PMC article. Review.
-
Therapeutic Potential of Intrabodies for Cancer Immunotherapy: Current Status and Future Directions.Antibodies (Basel). 2022 Jul 18;11(3):49. doi: 10.3390/antib11030049. Antibodies (Basel). 2022. PMID: 35892709 Free PMC article. Review.
-
Design of nanobody-based bispecific constructs by in silico affinity maturation and umbrella sampling simulations.Comput Struct Biotechnol J. 2022 Dec 16;21:601-613. doi: 10.1016/j.csbj.2022.12.021. eCollection 2023. Comput Struct Biotechnol J. 2022. PMID: 36659922 Free PMC article.
-
TLR2 and TLR9 Blockade Using Specific Intrabodies Inhibits Inflammation-Mediated Pancreatic Cancer Cell Growth.Antibodies (Basel). 2024 Feb 1;13(1):11. doi: 10.3390/antib13010011. Antibodies (Basel). 2024. PMID: 38390872 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;57:43–66. - PubMed
-
- Kang YW, Lee JE, Jung KH. KRAS targeting antibody synergizes anti-cancer activity of gemcitabine against pancreatic cancer. Cancer Lett. 2018;438:174–86. - PubMed
-
- Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017: clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15:1028–61. - PubMed
Grants and funding
- 2019YFA0905600/National Key Research and Development Project
- ZR2020ZD11/Major State Basic Research Development Program of the Natural Science Foundation of Shandong Province in China
- 81772633/National Natural Science Foundation of China
- 31470967/National Natural Science Foundation of China
- 19YFSLQY00110/Science and Technology Program of Tianjin, China
LinkOut - more resources
Full Text Sources
Other Literature Sources
