Secondary Structure-Driven Self-Assembly of Thiol-Reactive Polypept(o)ides
- PMID: 33830742
- PMCID: PMC8154267
- DOI: 10.1021/acs.biomac.1c00253
Secondary Structure-Driven Self-Assembly of Thiol-Reactive Polypept(o)ides
Abstract
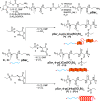
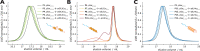
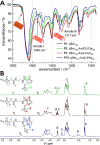
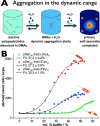
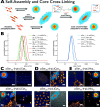
Secondary structure formation differentiates polypeptides from most of the other synthetic polymers, and the transitions from random coils to rod-like α-helices or β-sheets represent an additional parameter to direct self-assembly and the morphology of nanostructures. We investigated the influence of distinct secondary structures on the self-assembly of reactive amphiphilic polypept(o)ides. The individual morphologies can be preserved by core cross-linking via chemoselective disulfide bond formation. A series of thiol-responsive copolymers of racemic polysarcosine-block-poly(S-ethylsulfonyl-dl-cysteine) (pSar-b-p(dl)Cys), enantiopure polysarcosine-block-poly(S-ethylsulfonyl-l-cysteine) (pSar-b-p(l)Cys), and polysarcosine-block-poly(S-ethylsulfonyl-l-homocysteine) (pSar-b-p(l)Hcy) was prepared by N-carboxyanhydride polymerization. The secondary structure of the peptide segment varies from α-helices (pSar-b-p(l)Hcy) to antiparallel β-sheets (pSar-b-p(l)Cys) and disrupted β-sheets (pSar-b-p(dl)Cys). When subjected to nanoprecipitation, copolymers with antiparallel β-sheets display the strongest tendency to self-assemble, whereas disrupted β-sheets hardly induce aggregation. This translates to worm-like micelles, solely spherical micelles, or ellipsoidal structures, as analyzed by atomic force microscopy and cryogenic transmission electron microscopy, which underlines the potential of secondary structure-driven self-assembly of synthetic polypeptides.
Conflict of interest statement
The authors declare the following competing financial interest(s): Matthias Barz holds the patent Thiol-protected amino acid derivatives and uses thereof WO2015169908A1.
Figures
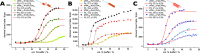
References
-
- Bonduelle C. Secondary Structures of Synthetic Polypeptide Polymers. Polym. Chem. 2018, 9, 1517–1529. 10.1039/C7PY01725A. - DOI
-
- Kricheldorf H. R.; Müller D. Secondary Structure of Peptides. Polym. Bull. 1983, 10, 513–520. 10.1007/BF00285369. - DOI
-
- Deng C.; Wu J.; Cheng R.; Meng F.; Klok H. A.; Zhong Z. Functional Polypeptide and Hybrid Materials: Precision Synthesis via α-Amino Acid N-Carboxyanhydride Polymerization and Emerging Biomedical Applications. Prog. Polym. Sci. 2014, 39, 330–364. 10.1016/j.progpolymsci.2013.10.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

