Human naive epiblast cells possess unrestricted lineage potential
- PMID: 33831366
- PMCID: PMC8189439
- DOI: 10.1016/j.stem.2021.02.025
Human naive epiblast cells possess unrestricted lineage potential
Abstract
Classic embryological experiments have established that the early mouse embryo develops via sequential lineage bifurcations. The first segregated lineage is the trophectoderm, essential for blastocyst formation. Mouse naive epiblast and derivative embryonic stem cells are restricted accordingly from producing trophectoderm. Here we show, in contrast, that human naive embryonic stem cells readily make blastocyst trophectoderm and descendant trophoblast cell types. Trophectoderm was induced rapidly and efficiently by inhibition of ERK/mitogen-activated protein kinase (MAPK) and Nodal signaling. Transcriptome comparison with the human embryo substantiated direct formation of trophectoderm with subsequent differentiation into syncytiotrophoblast, cytotrophoblast, and downstream trophoblast stem cells. During pluripotency progression lineage potential switches from trophectoderm to amnion. Live-cell tracking revealed that epiblast cells in the human blastocyst are also able to produce trophectoderm. Thus, the paradigm of developmental specification coupled to lineage restriction does not apply to humans. Instead, epiblast plasticity and the potential for blastocyst regeneration are retained until implantation.
Keywords: pluripotency, epiblast, embryonic stem cells, trophoblast, embryo, blastocyst, lineage segregation, differentiation.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.S. and G.G. are inventors on a patent application relating to human naive stem cells filed by the University of Cambridge.
Figures

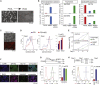


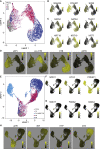


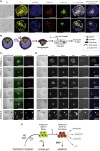
Comment in
-
The treasure inside human naive pluripotency, generation of trophectoderm and blastoids.Cell Stem Cell. 2021 Jun 3;28(6):985-987. doi: 10.1016/j.stem.2021.05.010. Cell Stem Cell. 2021. PMID: 34087157
References
-
- Adachi K., Nikaido I., Ohta H., Ohtsuka S., Ura H., Kadota M., Wakayama T., Ueda H.R., Niwa H. Context-dependent wiring of Sox2 regulatory networks for self-renewal of embryonic and trophoblast stem cells. Mol. Cell. 2013;52:380–392. - PubMed
-
- Beddington R.S.P., Robertson E.J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

