Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies
- PMID: 33831476
- PMCID: PMC8299409
- DOI: 10.1016/j.addr.2021.03.021
Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies
Abstract
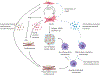
Cardiac fibrosis remains an unresolved problem in heart diseases. After initial injury, cardiac fibroblasts (CFs) are activated and subsequently differentiate into myofibroblasts (myoFbs) that are major mediator cells in the pathological remodeling. MyoFbs exhibit proliferative and secretive characteristics, and contribute to extracellular matrix (ECM) turnover, collagen deposition. The persistent functions of myoFbs lead to fibrotic scars and cardiac dysfunction. The anti-fibrotic treatment is hindered by the elusive mechanism of fibrosis and lack of specific targets on myoFbs. In this review, we will outline the progress of cardiac fibrosis and its contributions to the heart failure. We will also shed light on the role of myoFbs in the regulation of adverse remodeling. The communication between myoFbs and other cells that are involved in the heart injury and repair respectively will be reviewed in detail. Then, recently developed therapeutic strategies to treat fibrosis will be summarized such as i) chimeric antigen receptor T cell (CAR-T) therapy with an optimal target on myoFbs, ii) direct reprogramming from stem cells to quiescent CFs, iii) "off-target" small molecular drugs. The application of nano/micro technology will be discussed as well, which is involved in the construction of cell-based biomimic platforms and "pleiotropic" drug delivery systems.
Keywords: Cardiac fibroblast; Cardiac fibrosis; Drug delivery systems; Myocardial remodeling; Myofibroblasts; Reprogramming.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu SM, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MBC, Amer Heart Assoc Stat, S. Stroke Stat, Heart disease and stroke statistics-2016 update a report from the American Heart Association, Circulation 133 (2016) E38–E360. - PubMed
-
- Porter KE, Turner NA, Cardiac fibroblasts: at the heart of myocardial remodeling, Pharmacol. Ther. 123 (2009) 255–278. - PubMed
-
- Jellis C, Martin J, Narula J, Marwick TH, Assessment of nonischemic myocardial fibrosis, J. Am. Coll. Cardiol. 56 (2010) 89–97. - PubMed
-
- Hajipour MJ, Mehrani M, Abbasi SH, Amin A, Kassaian SE, Garbern JC, Caracciolo G, Zanganeh S, Chitsazan M, Aghaverdi H, Kamali Shahri SM, Ashkarran A, Raoufi M, Bauser-Heaton H, Zhang J, Muehlschlegel JD, Moore A, Lee RT, Wu JC, Serpooshan V, Mahmoudi M, Nanoscale technologies for prevention and treatment of heart failure: challenges and opportunities, Chem. Rev. 119 (2019) 11352–11390. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

