Environmentally relevant developmental methylmercury exposures alter neuronal differentiation in a human-induced pluripotent stem cell model
- PMID: 33831500
- PMCID: PMC8761391
- DOI: 10.1016/j.fct.2021.112178
Environmentally relevant developmental methylmercury exposures alter neuronal differentiation in a human-induced pluripotent stem cell model
Abstract
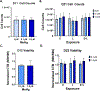
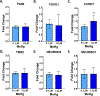
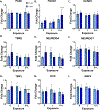
Developmental methylmercury (MeHg) exposure selectively targets the cerebral and cerebellar cortices, as seen by disruption of cytoarchitecture and glutamatergic (GLUergic) neuron hypoplasia. To begin to understand the mechanisms of this loss of GLUergic neurons, we aimed to develop a model of developmental MeHg neurotoxicity in human-induced pluripotent stem cells differentiating into cortical GLUergic neurons. Three dosing paradigms at 0.1 μM and 1.0 μM MeHg, which span different stages of neurodevelopment and reflect toxicologically relevant accumulation levels seen in human studies and mammalian models, were established. With these exposure paradigms, no changes were seen in commonly studied endpoints of MeHg toxicity, including viability, proliferation, and glutathione levels. However, MeHg exposure induced changes in mitochondrial respiration and glycolysis and in markers of neuronal differentiation. Our novel data suggests that GLUergic neuron hypoplasia seen with MeHg toxicity may be due to the partial inhibition of neuronal differentiation, given the increased expression of the early dorsal forebrain marker FOXG1 and corresponding decrease in expression on neuronal markers MAP2 and DCX and the deep layer cortical neuronal marker TBR1. Future studies should examine the persistent and latent functional effects of this MeHg-induced disruption of neuronal differentiation as well as transcriptomic and metabolomic alterations that may mediate MeHg toxicity.
Keywords: Developmental neurotoxicity; Glutamatergic neurons; Human-induced pluripotent stem cells; Methylmercury.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

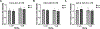




References
-
- Amin-Zaki L, Majeed MA, Elhassani SB, Clarkson TW, Greenwood MR, & Doherty RA (1979). Prenatal methylmercury poisoning. Clinical observations over five years. Am J Dis Child, 133(2), 172–177. - PubMed
-
- Aschner M, Ceccatelli S, Daneshian M, Fritsche E, Hasiwa N, Hartung T, Hogberg HT, Leist M, Li A, Mundi WR, Padilla S, Piersma AH, Bal-Price A, Seiler A, Westerink RH, Zimmer B. and Lein PJ (2017). Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use. ALTEX, 34(1), 49–74. doi:10.14573/altex.1604201 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

