Tear propagation in vaginal tissue under inflation
- PMID: 33831574
- PMCID: PMC11832074
- DOI: 10.1016/j.actbio.2021.03.065
Tear propagation in vaginal tissue under inflation
Abstract
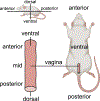
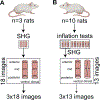
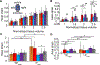
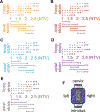
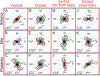
Vaginal tearing at childbirth is extremely common yet understudied despite the long-term serious consequences on women's health. The mechanisms of vaginal tearing remain unknown, and their knowledge could lead to the development of transformative prevention and treatment techniques for maternal injury. In this study, whole rat vaginas with pre-imposed elliptical tears oriented along the axial direction of the organs were pressurized using a custom-built inflation setup, producing large tear propagation. Large deformations of tears through propagation were analyzed, and nonlinear strains around tears were calculated using the digital image correlation technique. Second harmonic generation microscopy was used to examine collagen fiber organization in mechanically untested and tested vaginal specimens. Tears became increasingly circular under pressure, propagating slowly up to the maximum pressure and then more rapidly. Hoop strains were significantly larger than axial strains and displayed a region- and orientation-dependent response with tear propagation. Imaging revealed initially disorganized collagen fibers that aligned along the axial direction with increasing pressure. Fibers in the near-regions of tear tips aligned toward the hoop direction, hampering tear propagation. Changes in tear geometry, regional strains, and fiber orientation revealed the inherent toughening mechanisms of the vaginal tissue. STATEMENT OF SIGNIFICANCE: Women's reproductive health has historically been understudied despite alarming maternal injury and mortality rates in the world. Maternal injury and disability can be reduced by advancing our limited understanding of the large deformations experienced by women's reproductive organs. This manuscript presents, for the first time, the mechanics of tear propagation in vaginal tissue and changes to the underlying collagen microstructure near to and far from the tear. A novel inflation setup capable of maintaining the in vivo tubular geometry of the vagina while propagating a pre-imposed tear was developed. Toughening mechanisms of the vagina to propagation were examined through measurements of tear geometry, strain distributions, and reorientation of collagen fibers. This research draws from current advances in the engineering science and mechanics fields with the goal of improving maternal health care.
Keywords: Digital image correlation; Inflation; Maternal trauma; Second-harmonic generation imaging; Tear propagation; Vaginal tissue.
Copyright © 2021 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

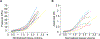



References
-
- Samuelsson E, Ladfors L, Lindblom BG, Hagberg H, A prospective observational study on tears during vaginal delivery: occurrences and risk factors, Acta Obstet. Gynecol. Scand. 81 (1) (2002) 44–49. - PubMed
-
- Hopkins LM, Caughey AB, Glidden DV, Laros RK, Racial/ethnic differences in perineal, vaginal and cervical lacerations, Am. J. Obstet. Gynecol 193 (2) (2005) 455–459. - PubMed
-
- Metcalfe A, Tohill S, Williams A, Haldon V, Brown L, Henry L, A pragmatic tool for the measurement of perineal tears, Br. J. Midwifery 10 (7) (2002) 412–417.
-
- Örnö AK, Maršál K, Herbst A, Ultrasonographic anatomy of perineal structures during pregnancy and immediately following obstetric injury, Ultrasound Obstet. Gynecol 32 (4) (2008) 527–534. - PubMed
-
- Fitzpatrick M, O’Herlihy C, Short-term and long-term effects of obstetric anal sphincter injury and their management, Curr. Opin. Obstet. Gynecol 17 (6) (2005) 605–610. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

