Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer's disease
- PMID: 33831607
- PMCID: PMC8161699
- DOI: 10.1016/j.arr.2021.101339
Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer's disease
Abstract
Objective: To investigate the effects of monoclonal antibodies against Aβ on cognition, function, amyloid PET and other biomarkers, as well as risk for amyloid-related imaging abnormalities (ARIA) and other adverse events, in Alzheimer's disease (AD).
Methods: Pubmed, Web of Science, ClinicalTrials.gov and gray literature were searched for phase III RCTs and random-effects meta-analyses were performed.
Results: Seventeen studies (12,585 patients) were included. Antibodies statistically improved the cognitive outcomes ADAS-Cog {SMD = -0.06 [95 % CI (-0.10; -0.02), I2 = 0%]} and MMSE {SMD = 0.05 [95 % CI (0.01; 0.09), I2 = 0%]} by small effect sizes, but did not improve the cognitive/functional measure CDR-SOB {SMD = -0.03 [95 % CI (-0.07; 0.01), I2 = 18 %]}. Moreover, antibodies decreased amyloid PET SUVR {SMD = -1.02 [95 % CI (-1.70; -0.34), I2 = 95 %]} and CSF p181-tau {SMD = -0.87 [95 % CI (-1.32; -0.43), I2 = 89 %]} by large effect sizes. They also increased risk for ARIA {RR = 4.30 [95 % CI (2.39; 7.77), I2 = 86 %]} by a large effect size. Antibody effects on reducing amyloid PET SUVR were correlated with their effects on improving ADAS-Cog (r = +0.68, p = 0.02). In subgroup analyses by individual drug, Aducanumab improved ADAS-Cog, CDR-SOB, ADCS-ADL by small effect sizes and decreased amyloid PET SUVR and CSF p181-tau by large effect sizes. Solanezumab improved ADAS-Cog and MMSE by small effect sizes, and increased (improved) CSF Aβ1-40 levels by a moderate effect size. Bapineuzumab, Gantenerumab and Crenezumab did not improve any clinical outcomes. Bapineuzumab and Gantenerumab decreased CSF p181-tau by a small and large effect size, respectively. All drugs except Solanezumab increased ARIA risk.
Conclusions: In this meta-analysis of phase III trials in AD, we found that monoclonal antibodies against Aβ induced clinical improvements of small effect sizes, biomarker improvements of large effect sizes, and increases in risk for the hallmark adverse event, ARIA, by a large effect size, when all drugs were pooled together. Among individual drugs, Aducanumab produced the most favorable effects followed by Solanezumab. These findings provide moderate support for the continuous development of anti-Aβ monoclonal antibodies as a treatment for AD.
Keywords: Alzheimer’s disease; Amyloid-beta; Meta-analysis; Monoclonal antibodies.
Published by Elsevier B.V.
Conflict of interest statement
Figures

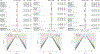
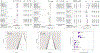
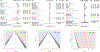
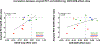
References
-
- Aisen PS, Cummings J, Doody R, Kramer L, Salloway S, Selkoe DJ, Sims J, Sperling RA, Vellas B, 2020. The Future of Anti-Amyloid Trials. J Prev Alzheimers Dis 7, 146–151. - PubMed
-
- Biogen, 2020. UPDATE ON FDA ADVISORY COMMITTEE’S MEETING ON ADUCANUMAB IN ALZHEIMER’S DISEASE.
-
- Biogen, 2021. BIOGEN AND EISAI ANNOUNCE FDA’S 3-MONTH EXTENSION OF REVIEW PERIOD FOR THE BIOLOGICS LICENSE APPLICATION FOR ADUCANUMAB.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

