Non-coding RNAs in pancreatic ductal adenocarcinoma: New approaches for better diagnosis and therapy
- PMID: 33831655
- PMCID: PMC8042452
- DOI: 10.1016/j.tranon.2021.101090
Non-coding RNAs in pancreatic ductal adenocarcinoma: New approaches for better diagnosis and therapy
Abstract
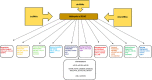
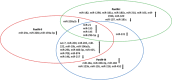
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies with a 5-year survival rate less than 8%, which has remained unchanged over the last 50 years. Early detection is particularly difficult due to the lack of disease-specific symptoms and a reliable biomarker. Multimodality treatment including chemotherapy, radiotherapy (used sparingly) and surgery has become the standard of care for patients with PDAC. Carbohydrate antigen 19-9 (CA 19-9) is the most common diagnostic biomarker; however, it is not specific enough especially for asymptomatic patients. Non-coding RNAs are often deregulated in human malignancies and shown to be involved in cancer-related mechanisms such as cell growth, differentiation, and cell death. Several micro, long non-coding and circular RNAs have been reported to date which are involved in PDAC. Aim of this review is to discuss the roles and functions of non-coding RNAs in diagnosis and treatments of PDAC.
Keywords: Circular RNA; Long non-coding RNA; MicroRNA; Non-coding RNAs; Pancreatic ductal adenocarcinoma.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest Authors declare that there is no financial/personal interest or belief that could affect the results, discussions or conclusions, which are reported in this work.
Figures

References
-
- Kocher H. Pancreatic cancer - symptoms, diagnosis and treatment. BMJ Best Practice. 2020 https://bestpractice.bmj.com/topics/en-gb/265
-
- Marzec J., Dayem Ullah A.Z., Pirrò S., Gadaleta E., Crnogorac-Jurcevic T., Lemoine N.R., Kocher H.M, Chelala C. The pancreatic expression database: 2018 update. Nucleic Acids Res. 2018;46(D1):D1107–D1110. 10.1093/nar/gkx955 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

