Impact of the subcutaneous formulations of trastuzumab and rituximab on efficiency and resource optimization in Spanish hospitals: H-Excelencia study
- PMID: 33832464
- PMCID: PMC8034176
- DOI: 10.1186/s12913-021-06277-8
Impact of the subcutaneous formulations of trastuzumab and rituximab on efficiency and resource optimization in Spanish hospitals: H-Excelencia study
Abstract
Background: Subcutaneous (SC) versus intravenous (IV) administration is advantageous in terms of patient convenience and hospital efficiency. This study aimed to compare the effect of optimizing the processes involved in SC versus IV administration of rituximab and trastuzumab on hospital capacity and service quality.
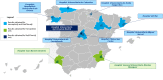
Methods: This cross-sectional resource utilization study interviewed oncologists, hematologists, nurses, and pharmacists from 10 hospitals in Spain to estimate changes in processes associated with conversion from IV to SC rituximab and trastuzumab, based on clinical experience and healthcare use from administrative databases.
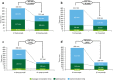
Results: Efficient use of SC formulations increased the monthly capacity for parenteral administration by 3.35% (potentially increasable by 5.75% with maximum possible conversion according to the product label). The weekly capacity for hospital pharmacy treatment preparation increased by 7.13% due to conversion to SC formulation and by 9.33% due to transferring SC preparation to the cancer treatment unit (potentially increasable by 12.16 and 14.10%, respectively). Monthly hospital time decreased by 33% with trastuzumab and 47% with rituximab. In a hypothetical hospital, in which all processes for efficient use of SC rituximab and/or trastuzumab were implemented and all eligible patients received SC formulations, the estimated monthly capacity for preparation and administration increased by 23.1% and estimated hospital times were reduced by 60-66%.
Conclusions: Conversion of trastuzumab and rituximab to SC administration could improve the efficiency of hospitals and optimize internal resource management processes, potentially increasing care capacity and improving the quality of care by reducing time spent by patients at hospitals.
Keywords: Efficiency indicators; Healthcare capacity; Healthcare quality; Intravenous; Resource optimization; Subcutaneous.
Conflict of interest statement
MRA-S reports speakers’ fees from Roche, Shire, Grifols, Janssen, GSK, Merck, Abbvie, Leo-Pharma and Vertex, and non-financial support from Roche and Janssen. JMGdeC reports speakers’ fees from MSD, Roche, Alexion and Novartis, and travel expenses from MSD. JAL has received speakers’ fees from ROCHE. CMC reports speakers’ fees and travel expenses from Roche. AS has received honoraria and speakers’ fees from Roche, Jannsen and Celgene and travel expenses from Roche and Celgene. All other authors declare that they have no competing interest.
Figures
References
-
- European Medicines Agency . MabThera (rituximab) [summary of product characteristics] 2008.
-
- European Medicines Agency . Herceptin (trastuzumab) [summary of product characteristics] 2010.
-
- Davies A, Berge C, Boehnke A, Dadabhoy A, Lugtenburg P, Rule S, Rummel M, McIntyre C, Smith R, Badoux X. Subcutaneous rituximab for the treatment of B-cell hematologic malignancies: a review of the scientific rationale and clinical development. Adv Ther. 2017;34(10):2210–2231. doi: 10.1007/s12325-017-0610-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

