Allogeneic transplant procurement in the times of COVID-19: Quality report from the central European cryopreservation site
- PMID: 33832504
- PMCID: PMC8027980
- DOI: 10.1186/s12967-021-02810-9
Allogeneic transplant procurement in the times of COVID-19: Quality report from the central European cryopreservation site
Abstract
Background: Because of limitations of transportation imposed by the COVID-19 pandemic, current recommendation calls for cryopreservation of allogeneic stem cell transplants before patient conditioning. A single cell therapy laboratory was selected to function as the central cryopreservation hub for all European registry donor transplants intended for the Australian-Pacific region. We examined properties of these transplants to ascertain how quality is maintained.
Methods: We analyzed 100 pandemic-related allogeneic mobilized blood-derived stem cell apheresis products generated at 30 collection sites throughout Europe, shipped to and cryopreserved at our center between April and November of 2020. Products were shipped in the cool, subsequently frozen with DMSO as cryoprotectant. Irrespective of origin, all products were frozen within the prescribed shelf-life of 72 h.
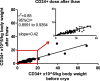
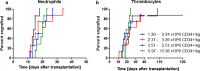
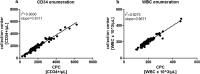
Results: Prior to cryopreservation, viable stem cell and leukocyte count according to the collection site and our reference laboratory were highly concordant (r2 = 0.96 and 0.93, respectively) and viability was > 90% in all instances. Median nominal post-thaw recovery of viable CD34+ cells was 42%. Weakly associated with poorer CD34+ cell recovery was higher leukocyte concentration, but not time lag between apheresis or addition of cryopreservant, respectively, and start of freezing. The correlation between pre- and post-thaw CD34+ cell dose was high (r2 = 0.85), hence predictable. Neutrophil and platelet engraftment were prompt with no evidence of dose dependency within the range of administered cell doses (1.31-15.56 × 106 CD34+ cells/kg).
Conclusions: General cryopreservation of allogeneic stem cell transplants is feasible. While more than half of the CD34+ cell content is lost, the remaining stem cells ensure timely engraftment.
Keywords: CD34+ cell enumeration; Engraftment; Graft; Graft quality; Hematopoietic stem cell; Post-thaw recovery; Quality control; Stem cell dose; Stem cell enumeration; Transplant logistics.
Conflict of interest statement
V.S. received support for attending meetings and/or travel from Pfizer and Gilead, P.B. receives institutional research grants from Riemser, Medac and Neovii, holds patent and receives Royalties from Medac, is member of Speakers bureau at Novartis and Amgen and member of Advisory boards at Amgen, Novartis and Medac. H.B. receives institutional research funding from Sandoz-Hexal, receives Licensing Fees and Royalties from Medac, is a member of the Speakers Bureaus and/or Advisory Boards of Novartis, Celgene, Boehringer Ingelheim, Miltenyi Biotech and Medac. The remaining authors declare no competing financial interests.
Figures


References
-
- Ardura M, Hartley D, Dandoy C, et al. Addressing the Impact of the Coronavirus Disease 2019 (COVID-19) pandemic on hematopoietic cell transplantation: learning networks as a means for sharing best practices. Biol Blood Marrow Transplant. 2020;26:e147–e160. doi: 10.1016/j.bbmt.2020.04.018. - DOI - PMC - PubMed
-
- EBMT recommendations. https://www.ebmt.org/sites/default/files/2020-06/EBMT%20COVID-19%20guide.... Accessed 1 Feb 2021.
-
- WMDA recommendations. https://share.wmda.info/pages/viewpage.action?pageId=344866320#/. Accessed 1 Feb 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

