Corneal confocal microscopy differentiates inflammatory from diabetic neuropathy
- PMID: 33832507
- PMCID: PMC8033689
- DOI: 10.1186/s12974-021-02130-1
Corneal confocal microscopy differentiates inflammatory from diabetic neuropathy
Abstract
Background: Immune-mediated neuropathies, such as chronic inflammatory demyelinating polyneuropathy (CIDP) are treatable neuropathies. Among individuals with diabetic neuropathy, it remains a challenge to identify those individuals who develop CIDP. Corneal confocal microscopy (CCM) has been shown to detect corneal nerve fiber loss and cellular infiltrates in the sub-basal layer of the cornea. The objective of the study was to determine whether CCM can distinguish diabetic neuropathy from CIDP and whether CCM can detect CIDP in persons with coexisting diabetes.
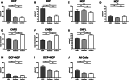
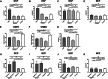
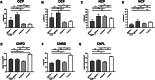
Methods: In this multicenter, case-control study, participants with CIDP (n = 55) with (n = 10) and without (n = 45) diabetes; participants with diabetes (n = 58) with (n = 28) and without (n = 30) diabetic neuropathy, and healthy controls (n = 58) underwent CCM. Corneal nerve fiber density (CNFD), corneal nerve fiber length (CNFL), corneal nerve branch density (CNBD), and dendritic and non-dendritic cell density, with or without nerve fiber contact were quantified.
Results: Dendritic cell density in proximity to corneal nerve fibers was significantly higher in participants with CIDP with and without diabetes compared to participants with diabetic neuropathy and controls. CNFD, CNFL, and CNBD were equally reduced in participants with CIDP, diabetic neuropathy, and CIDP with diabetes.
Conclusions: An increase in dendritic cell density identifies persons with CIDP. CCM may, therefore, be useful to differentiate inflammatory from non-inflammatory diabetic neuropathy.
Keywords: Chronic inflammatory demyelinating neuropathy; Diabetes mellitus; corneal confocal microscopy.
Conflict of interest statement
Fleischer (none), Lee (none), Hinrichs (none), Petropoulos (none), Erdlenbruch (none), Malik served on the scientific advisory board and/or received speaker honoraria or travel funding from Biogen Idec, Merck, Pfizer, Novo Nordisk. Hartung received personal fees for consulting, serving on steering, and data monitoring committees from Bayer Healthcare, Biogen, Celgene Receptos, CSL Behring, GeNeuro, MedImmune, Merck, Novartis, Octapharma, Roche, Sanofi Genzyme, and Teva. Kieseier (none). Kleinschnitz (none), Stettner: served on the scientific advisory and/or received speaker honoraria or travel funding from UCB, Biogen Idec; Grifols, Genzyme, Roche, Merck, Novartis, Octapharma, CSL Behring, Sanofi-Aventis, TEVA, and Bayer.
Figures
Similar articles
-
Corneal confocal microscopy detects small fiber damage in chronic inflammatory demyelinating polyneuropathy (CIDP).J Peripher Nerv Syst. 2014 Dec;19(4):322-7. doi: 10.1111/jns.12098. J Peripher Nerv Syst. 2014. PMID: 25582791 Clinical Trial.
-
The Utility of Corneal Nerve Fractal Dimension Analysis in Peripheral Neuropathies of Different Etiology.Transl Vis Sci Technol. 2020 Aug 28;9(9):43. doi: 10.1167/tvst.9.9.43. eCollection 2020 Aug. Transl Vis Sci Technol. 2020. PMID: 32934893 Free PMC article.
-
Corneal confocal microscopy as a non-invasive test to assess diabetic peripheral neuropathy.Diabetes Res Clin Pract. 2018 Feb;136:85-92. doi: 10.1016/j.diabres.2017.11.026. Epub 2017 Dec 6. Diabetes Res Clin Pract. 2018. PMID: 29221815
-
Corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy: A systematic review and meta-analysis.J Diabetes Investig. 2022 Jan;13(1):134-147. doi: 10.1111/jdi.13643. Epub 2021 Aug 27. J Diabetes Investig. 2022. PMID: 34351711 Free PMC article.
-
Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy.Curr Diab Rep. 2013 Aug;13(4):488-99. doi: 10.1007/s11892-013-0390-z. Curr Diab Rep. 2013. PMID: 23666893 Review.
Cited by
-
Corneal Confocal Microscopy to Image Small Nerve Fiber Degeneration: Ophthalmology Meets Neurology.Front Pain Res (Lausanne). 2021 Aug 19;2:725363. doi: 10.3389/fpain.2021.725363. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295436 Free PMC article. Review.
-
Corneal inflammatory cell infiltration predicts disease activity in chronic inflammatory demyelinating polyneuropathy.Sci Rep. 2021 Jul 26;11(1):15150. doi: 10.1038/s41598-021-94605-7. Sci Rep. 2021. PMID: 34312451 Free PMC article.
-
Corneal nerve fiber involvement in chronic inflammatory demyelinating polyneuropathy.Neurol Sci. 2023 Jul;44(7):2509-2516. doi: 10.1007/s10072-023-06711-1. Epub 2023 Mar 1. Neurol Sci. 2023. PMID: 36856905
-
Corneal immune cells as a biomarker of inflammation in multiple sclerosis: a longitudinal study.Ther Adv Neurol Disord. 2023 Oct 29;16:17562864231204974. doi: 10.1177/17562864231204974. eCollection 2023. Ther Adv Neurol Disord. 2023. PMID: 37915502 Free PMC article.
-
Clinical associations of corneal neuromas with ocular surface diseases.Neural Regen Res. 2024 Jan;19(1):140-147. doi: 10.4103/1673-5374.375308. Neural Regen Res. 2024. PMID: 37488855 Free PMC article. Review.
References
-
- Querol L, Crabtree M, Herepath M, Priedane E, Viejo Viejo I, Agush S, Sommerer P. 2020. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol - PubMed
-
- Liberatore G, Manganelli F, Doneddu PE, Cocito D, Fazio R, Briani C, Filosto M, Benedetti L, Mazzeo A, Antonini G, Cosentino G, Jann S, Cortese A, Marfia GA, Clerici AM, Siciliano G, Carpo M, Luigetti M, Lauria G, Rosso T, Cavaletti G, Santoro L, Peci E, Tronci S, Ruiz M, Cotti Piccinelli S, Schenone A, Leonardi L, Toscano A, Mataluni G, Spina E, Gentile L, Nobile-Orazio E. 2020. Chronic inflammatory demyelinating polyradiculoneuropathy: can a diagnosis be made in patients not fulfilling electrodiagnostic criteria? Eur J Neurol - PubMed
-
- Stino AM, Naddaf E, Dyck PJ, Dyck PJB. 2020. Chronic inflammatory demyelinating polyradiculoneuropathy-Diagnostic pitfalls and treatment approach. Muscle Nerve - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

