Options for imaging cellular therapeutics in vivo: a multi-stakeholder perspective
- PMID: 33832818
- PMCID: PMC9344904
- DOI: 10.1016/j.jcyt.2021.02.005
Options for imaging cellular therapeutics in vivo: a multi-stakeholder perspective
Abstract
Cell-based therapies have been making great advances toward clinical reality. Despite the increase in trial activity, few therapies have successfully navigated late-phase clinical trials and received market authorization. One possible explanation for this is that additional tools and technologies to enable their development have only recently become available. To support the safety evaluation of cell therapies, the Health and Environmental Sciences Institute Cell Therapy-Tracking, Circulation and Safety Committee, a multisector collaborative committee, polled the attendees of the 2017 International Society for Cell & Gene Therapy conference in London, UK, to understand the gaps and needs that cell therapy developers have encountered regarding safety evaluations in vivo. The goal of the survey was to collect information to inform stakeholders of areas of interest that can help ensure the safe use of cellular therapeutics in the clinic. This review is a response to the cellular imaging interests of those respondents. The authors offer a brief overview of available technologies and then highlight the areas of interest from the survey by describing how imaging technologies can meet those needs. The areas of interest include imaging of cells over time, sensitivity of imaging modalities, ability to quantify cells, imaging cellular survival and differentiation and safety concerns around adding imaging agents to cellular therapy protocols. The Health and Environmental Sciences Institute Cell Therapy-Tracking, Circulation and Safety Committee believes that the ability to understand therapeutic cell fate is vital for determining and understanding cell therapy efficacy and safety and offers this review to aid in those needs. An aim of this article is to share the available imaging technologies with the cell therapy community to demonstrate how these technologies can accomplish unmet needs throughout the translational process and strengthen the understanding of cellular therapeutics.
Keywords: biodistribution; cell therapy; fate; in vivo tracking; non-invasive; safety.
Copyright © 2021 International Society for Cell & Gene Therapy. Published by Elsevier Inc. All rights reserved.
Figures

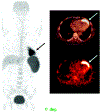
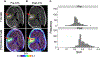


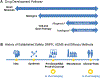
References
-
- European Medicines Agency. Advanced therapy medicinal products: overview. https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-...; 2020. [accessed 20 March 2020].
-
- June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018;359:1361–5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

