Aberrant TGF-β1 signaling activation by MAF underlies pathological lens growth in high myopia
- PMID: 33833231
- PMCID: PMC8032689
- DOI: 10.1038/s41467-021-22041-2
Aberrant TGF-β1 signaling activation by MAF underlies pathological lens growth in high myopia
Erratum in
-
Author Correction: Aberrant TGF-β1 signaling activation by MAF underlies pathological lens growth in high myopia.Nat Commun. 2022 Dec 20;13(1):7834. doi: 10.1038/s41467-022-35562-1. Nat Commun. 2022. PMID: 36539467 Free PMC article. No abstract available.
Abstract
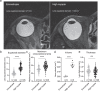
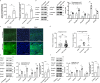
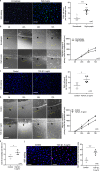
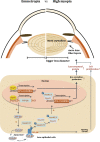
High myopia is a leading cause of blindness worldwide. Myopia progression may lead to pathological changes of lens and affect the outcome of lens surgery, but the underlying mechanism remains unclear. Here, we find an increased lens size in highly myopic eyes associated with up-regulation of β/γ-crystallin expressions. Similar findings are replicated in two independent mouse models of high myopia. Mechanistic studies show that the transcription factor MAF plays an essential role in up-regulating β/γ-crystallins in high myopia, by direct activation of the crystallin gene promoters and by activation of TGF-β1-Smad signaling. Our results establish lens morphological and molecular changes as a characteristic feature of high myopia, and point to the dysregulation of the MAF-TGF-β1-crystallin axis as an underlying mechanism, providing an insight for therapeutic interventions.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

