Interleukin-6 as an enhancer of anti-angiogenic therapy for ovarian clear cell carcinoma
- PMID: 33833265
- PMCID: PMC8032732
- DOI: 10.1038/s41598-021-86913-9
Interleukin-6 as an enhancer of anti-angiogenic therapy for ovarian clear cell carcinoma
Abstract
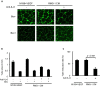
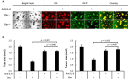
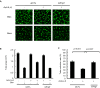
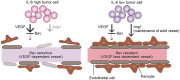
Ovarian clear cell carcinoma (OCCC) is a subtype of epithelial ovarian cancer (EOC) that is associated with elevated interleukin-6 (IL-6) expression, resistance to chemotherapy, and increased mortality. Although bevacizumab (Bev) is a widely used anti-angiogenic agent for EOC, the efficacy of Bev and the role of IL-6 in modulating angiogenesis in OCCC are unknown. We performed tube formation assays using human umbilical vein endothelial cells (HUVEC) cultured in OCCC cell-conditioned medium and using cells directly co-cultured with OCCC cells. We observed that IL-6 inhibition significantly mitigated the ability of Bev to impede tube formation in both cases. Furthermore, IL-6 blockade disrupted the anti-angiogenic efficacy of Bev and its concomitant anti-tumor activity. In addition, IL-6 inhibition resulted in a significant increase in angiopoietin-1 (Ang1) secretion and decreased vascular endothelial growth factor (VEGF) expression. Clinical specimens also exhibited this reciprocal relationship between IL-6 and Ang1 expression. Finally, depletion of Ang1 abrogated the effects of IL-6 inhibition on Bev activity, demonstrating that IL-6 supports the anti-angiogenic activity of Bev by suppressing Ang1 expression and promoting dependence on VEGF for angiogenesis. Altogether, our data suggest that OCCC tumors with high IL-6 levels are candidates for Bev therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Efficacy of trebananib (AMG 386) in treating epithelial ovarian cancer.Expert Opin Pharmacother. 2016;17(6):853-60. doi: 10.1517/14656566.2016.1161027. Epub 2016 Mar 21. Expert Opin Pharmacother. 2016. PMID: 26933765 Review.
-
Status of alternative angiogenic pathways in glioblastoma resected under and after bevacizumab treatment.Brain Tumor Pathol. 2024 Apr;41(2):61-72. doi: 10.1007/s10014-024-00481-0. Epub 2024 Apr 15. Brain Tumor Pathol. 2024. PMID: 38619734 Free PMC article.
-
Xiaotan Sanjie decoction inhibits angiogenesis in gastric cancer through Interleukin-8-linked regulation of the vascular endothelial growth factor pathway.J Ethnopharmacol. 2016 Aug 2;189:230-7. doi: 10.1016/j.jep.2016.05.043. Epub 2016 May 17. J Ethnopharmacol. 2016. PMID: 27224240
-
Continuous Administration of Anti-VEGFA Antibody Upregulates PAI-1 Secretion from Ovarian Cancer Cells via miR-143-3p Downregulation.Mol Cancer Res. 2023 Oct 2;21(10):1093-1106. doi: 10.1158/1541-7786.MCR-23-0015. Mol Cancer Res. 2023. PMID: 37327051
-
Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer.Int J Mol Sci. 2017 Sep 14;18(9):1967. doi: 10.3390/ijms18091967. Int J Mol Sci. 2017. PMID: 28906427 Free PMC article. Review.
Cited by
-
In vitro Evaluation of the Cytotoxic Effects of a Recombinant form of the Soluble Mutant IL-6 Receptor on an Ovarian Cancer Cell Line.Iran J Biotechnol. 2025 Jan 1;23(1):e3953. doi: 10.30498/ijb.2025.467140.3953. eCollection 2025 Jan. Iran J Biotechnol. 2025. PMID: 40463947 Free PMC article.
-
IL-6 Polymorphism as a Predisposing Genetic Factor for Gestational Diabetes or Preeclampsia Development in Pregnancy with Obesity in Relation to VEGF and VEGFF Receptor Gene Expression Modalities.Diagnostics (Basel). 2024 Jun 6;14(11):1206. doi: 10.3390/diagnostics14111206. Diagnostics (Basel). 2024. PMID: 38893732 Free PMC article.
-
Retrospective analysis of treatment and prognosis for clear cell carcinoma of the uterine cervix: 15-year experience at a single institution.J Obstet Gynaecol Res. 2025 Apr;51(4):e16300. doi: 10.1111/jog.16300. J Obstet Gynaecol Res. 2025. PMID: 40268756 Free PMC article.
-
Therapeutic Strategies Focused on Cancer-Associated Hypercoagulation for Ovarian Clear Cell Carcinoma.Cancers (Basel). 2022 Apr 24;14(9):2125. doi: 10.3390/cancers14092125. Cancers (Basel). 2022. PMID: 35565252 Free PMC article. Review.
-
Interleukin-6 serves as a critical factor in various cancer progression and therapy.Med Oncol. 2024 Jun 20;41(7):182. doi: 10.1007/s12032-024-02422-5. Med Oncol. 2024. PMID: 38900329 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

