Robenacoxib shows efficacy for the treatment of chronic degenerative joint disease-associated pain in cats: a randomized and blinded pilot clinical trial
- PMID: 33833276
- PMCID: PMC8032665
- DOI: 10.1038/s41598-021-87023-2
Robenacoxib shows efficacy for the treatment of chronic degenerative joint disease-associated pain in cats: a randomized and blinded pilot clinical trial
Abstract
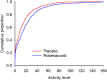
The main objective of this pilot clinical trial was to evaluate outcome measures for the assessment of the nonsteroidal anti-inflammatory drug (NSAID) robenacoxib in cats with degenerative joint disease-associated pain (DJD-pain). Otherwise healthy cats (n = 109) with DJD-pain entered a parallel group, randomized, blinded clinical trial. Cats received placebo (P) or robenacoxib (R) for two consecutive 3-week periods. Treatment groups were PP, RR, and RP. Actimetry and owner-assessment data were collected. Data were analyzed using mixed-effects and generalized mixed-effects linear models. Activity data showed high within-cat and between-cat variability, and 82.4% of the values were zero. Compared to placebo, mean total activity was higher (5.7%) in robenacoxib-treated cats (p = 0.24); for the 80th percentile of activity, more robenacoxib-treated cats had a > 10% increase in activity after 3 (p = 0.046) and 6 weeks (p = 0.026). Robenacoxib treatment significantly decreased owner-assessed disability, (p = 0.01; 49% reduction in disability; effect size ~ 0.3), and improved temperament (p = 0.0039) and happiness (p = 0.021) after 6 weeks. More robenacoxib-treated cats were successes at 6 weeks (p = 0.018; NNT: 3.8). Adverse effect frequencies were similar across groups. Results identified suitable endpoints for confirmatory studies, while also indicating efficacy of robenacoxib in cats with DJD-pain.
Conflict of interest statement
The study was funded initially by Novartis Animal Health and later (after its acquisition) by Elanco Animal Health, which manufacture and distribute robenacoxib (Onsior) tablets. DA was a graduate student in the TRiP program at the time of the study and is now employed by Elanco Animal Health. JNK and SBK are employed and RSP was previously employed by Elanco Animal Health. BDXL and SCB have received consultancy fees and honoraria for lecturing (including reimbursement for travel and accommodations) on behalf of Novartis Animal Health and Elanco Animal Health. MEG has no conflicts of interest to declare.
Figures


Similar articles
-
Pharmacology, safety, efficacy and clinical uses of the COX-2 inhibitor robenacoxib.J Vet Pharmacol Ther. 2022 Jul;45(4):325-351. doi: 10.1111/jvp.13052. Epub 2022 Apr 22. J Vet Pharmacol Ther. 2022. PMID: 35460083 Free PMC article. Review.
-
Clinical safety of robenacoxib in feline osteoarthritis: results of a randomized, blinded, placebo-controlled clinical trial.J Feline Med Surg. 2016 Aug;18(8):632-42. doi: 10.1177/1098612X15590870. Epub 2015 Jun 9. J Feline Med Surg. 2016. PMID: 26058587 Free PMC article. Clinical Trial.
-
Robenacoxib versus meloxicam for the control of peri-operative pain and inflammation associated with orthopaedic surgery in cats: a randomised clinical trial.BMC Vet Res. 2015 Mar 26;11:79. doi: 10.1186/s12917-015-0391-z. BMC Vet Res. 2015. PMID: 25880535 Free PMC article. Clinical Trial.
-
Comparison of oral robenacoxib and ketoprofen for the treatment of acute pain and inflammation associated with musculoskeletal disorders in cats: a randomised clinical trial.Vet J. 2012 Aug;193(2):397-403. doi: 10.1016/j.tvjl.2012.02.008. Epub 2012 Mar 18. Vet J. 2012. PMID: 22430026 Clinical Trial.
-
DJD-associated pain in cats: what can we do to promote patient comfort?J Feline Med Surg. 2010 Mar;12(3):200-12. doi: 10.1016/j.jfms.2010.01.003. J Feline Med Surg. 2010. PMID: 20193911 Free PMC article. Review.
Cited by
-
Evaluation of a nutritional supplement for the alleviation of pain associated with feline degenerative joint disease: a prospective, randomized, stratified, double-blind, placebo-controlled clinical trial.J Feline Med Surg. 2022 Oct;24(10):962-974. doi: 10.1177/1098612X211053484. Epub 2021 Nov 1. J Feline Med Surg. 2022. PMID: 34719996 Free PMC article.
-
Osteoarthritis in cats: what we know, and mostly, what we don't know. . . yet.J Feline Med Surg. 2025 Jul;27(7):1098612X251347999. doi: 10.1177/1098612X251347999. Epub 2025 Jul 20. J Feline Med Surg. 2025. PMID: 40685570 Free PMC article. Review.
-
2024 ISFM and AAFP consensus guidelines on the long-term use of NSAIDs in cats.J Feline Med Surg. 2024 Apr;26(4):1098612X241241951. doi: 10.1177/1098612X241241951. J Feline Med Surg. 2024. PMID: 38587872 Free PMC article.
-
Pharmacology, safety, efficacy and clinical uses of the COX-2 inhibitor robenacoxib.J Vet Pharmacol Ther. 2022 Jul;45(4):325-351. doi: 10.1111/jvp.13052. Epub 2022 Apr 22. J Vet Pharmacol Ther. 2022. PMID: 35460083 Free PMC article. Review.
-
Development of Two Innovative Performance-Based Objective Measures in Feline Osteoarthritis: Their Reliability and Responsiveness to Firocoxib Analgesic Treatment.Int J Mol Sci. 2022 Oct 4;23(19):11780. doi: 10.3390/ijms231911780. Int J Mol Sci. 2022. PMID: 36233085 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

