Predicting the clinical management of skin lesions using deep learning
- PMID: 33833293
- PMCID: PMC8032721
- DOI: 10.1038/s41598-021-87064-7
Predicting the clinical management of skin lesions using deep learning
Abstract
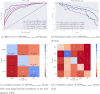
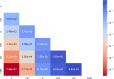
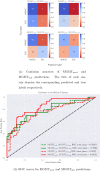
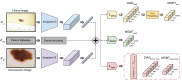
Automated machine learning approaches to skin lesion diagnosis from images are approaching dermatologist-level performance. However, current machine learning approaches that suggest management decisions rely on predicting the underlying skin condition to infer a management decision without considering the variability of management decisions that may exist within a single condition. We present the first work to explore image-based prediction of clinical management decisions directly without explicitly predicting the diagnosis. In particular, we use clinical and dermoscopic images of skin lesions along with patient metadata from the Interactive Atlas of Dermoscopy dataset (1011 cases; 20 disease labels; 3 management decisions) and demonstrate that predicting management labels directly is more accurate than predicting the diagnosis and then inferring the management decision ([Formula: see text] and [Formula: see text] improvement in overall accuracy and AUROC respectively), statistically significant at [Formula: see text]. Directly predicting management decisions also considerably reduces the over-excision rate as compared to management decisions inferred from diagnosis predictions (24.56% fewer cases wrongly predicted to be excised). Furthermore, we show that training a model to also simultaneously predict the seven-point criteria and the diagnosis of skin lesions yields an even higher accuracy (improvements of [Formula: see text] and [Formula: see text] in overall accuracy and AUROC respectively) of management predictions. Finally, we demonstrate our model's generalizability by evaluating on the publicly available MClass-D dataset and show that our model agrees with the clinical management recommendations of 157 dermatologists as much as they agree amongst each other.
Conflict of interest statement
G.H. serves as a Scientific Advisor to Triage Technologies Inc., Toronto, Canada, where J.K. and G.H. are minor shareholders (
Figures


References
-
- Bakheet S. An SVM framework for malignant melanoma detection based on optimized HOG features. Computation. 2017;5:4. doi: 10.3390/computation5010004. - DOI
-
- Grzesiak-Kopeć, K., Nowak, L. & Ogorzałek, M. Automatic diagnosis of melanoid skin lesions using machine learning methods. In Rutkowski, L. et al. (eds.) International Conference on Artificial Intelligence and Soft Computing, 577–585 (Springer, Cham, 2015). 10.1007/978-3-319-19324-3_51.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

