Anthracene induces oxidative stress and activation of antioxidant and detoxification enzymes in Ulva lactuca (Chlorophyta)
- PMID: 33833321
- PMCID: PMC8032757
- DOI: 10.1038/s41598-021-87147-5
Anthracene induces oxidative stress and activation of antioxidant and detoxification enzymes in Ulva lactuca (Chlorophyta)
Abstract
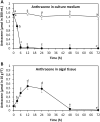
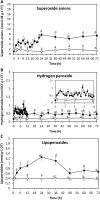
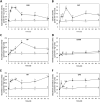
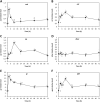
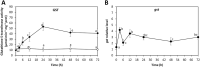
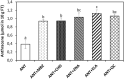
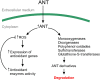
In order to analyze whether the marine macroalga Ulva lactuca can absorb and metabolize anthracene (ANT), the alga was cultivated with 5 µM ANT for 0-72 h, and the level of ANT was detected in the culture medium, and in the alga. The level of ANT rapidly decreased in the culture medium reaching a minimal level at 6 h, and rapidly increased in the alga reaching a maximal level at 12 h and then decreased to reach a minimal level at 48 h of culture. In addition, ANT induced an increase in hydrogen peroxide that remained until 72 h and a higher increase in superoxide anions that reach a maximal level at 24 h and remained unchanged until 72 h, indicating that ANT induced an oxidative stress condition. ANT induced an increase in lipoperoxides that reached a maximal level at 24 h and decreased at 48 h indicating that oxidative stress caused membrane damage. The activity of antioxidant enzymes SOD, CAT, AP, GR and GP increased in the alga treated with ANT whereas DHAR remained unchanged. The level of transcripts encoding these antioxidant enzymes increased and those encoding DHAR did not change. Inhibitors of monooxygenases, dioxygenases, polyphenol oxidases, glutathione-S-transferases and sulfotransferases induced an increase in the level of ANT in the alga cultivated for 24 h. These results strongly suggest that ANT is rapidly absorbed and metabolized in U. lactuca and the latter involves Phase I and II metabolizing enzymes.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Huang XD, Dixon DG, Greenberg BM. Impact of UV radiation and photomodification on the toxicity of PAHs to the higher plant Lemna gibba (duckweed) Environ. Toxicol. Chem. 1993;12:1067–1077.
-
- Carranza DM, et al. Socio-environmental conflicts: An underestimated threat to biodiversity conservation in Chile. Environ. Sci. Pol. 2020;110:46–59. doi: 10.1016/j.envsci.2020.04.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

