Actin cytoskeletal inhibitor 19,20-epoxycytochalasin Q sensitizes yeast cells lacking ERG6 through actin-targeting and secondarily through disruption of lipid homeostasis
- PMID: 33833332
- PMCID: PMC8032726
- DOI: 10.1038/s41598-021-87342-4
Actin cytoskeletal inhibitor 19,20-epoxycytochalasin Q sensitizes yeast cells lacking ERG6 through actin-targeting and secondarily through disruption of lipid homeostasis
Abstract
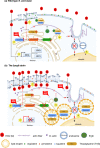
Repetitive uses of antifungals result in a worldwide crisis of drug resistance; therefore, natural fungicides with minimal side-effects are currently sought after. This study aimed to investigate antifungal property of 19, 20-epoxycytochalasin Q (ECQ), derived from medicinal mushroom Xylaria sp. BCC 1067 of tropical forests. In a model yeast Saccharomyces cerevisiae, ECQ is more toxic in the erg6∆ strain, which has previously been shown to allow higher uptake of many hydrophilic toxins. We selected one pathway to study the effects of ECQ at very high levels on transcription: the ergosterol biosynthesis pathway, which is unlikely to be the primary target of ECQ. Ergosterol serves many functions that cholesterol does in human cells. ECQ's transcriptional effects were correlated with altered sterol and triacylglycerol levels. In the ECQ-treated Δerg6 strain, which presumably takes up far more ECQ than the wild-type strain, there was cell rupture. Increased actin aggregation and lipid droplets assembly were also found in the erg6∆ mutant. Thereby, ECQ is suggested to sensitize yeast cells lacking ERG6 through actin-targeting and consequently but not primarily led to disruption of lipid homeostasis. Investigation of cytochalasins may provide valuable insight with potential biopharmaceutical applications in treatments of fungal infection, cancer or metabolic disorder.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Inhibition of cell cycle-dependent hyphal and biofilm formation by a novel cytochalasin 19,20‑epoxycytochalasin Q in Candida albicans.Sci Rep. 2023 Jun 15;13(1):9724. doi: 10.1038/s41598-023-36191-4. Sci Rep. 2023. PMID: 37322086 Free PMC article.
-
An actin depolymerizing agent 19,20-epoxycytochalasin Q of Xylaria sp. BCC 1067 enhanced antifungal action of azole drugs through ROS-mediated cell death in yeast.Microbiol Res. 2021 Feb;243:126646. doi: 10.1016/j.micres.2020.126646. Epub 2020 Nov 9. Microbiol Res. 2021. PMID: 33227681
-
[Regulation role of sterol C-24 methyltransferase and sterol C-8 isomerase in the ergosterol biosynthesis of Saccharomyces cerevisiae].Wei Sheng Wu Xue Bao. 2009 Aug;49(8):1063-8. Wei Sheng Wu Xue Bao. 2009. PMID: 19835168 Chinese.
-
Biochemical and physiological effects of sterol alterations in yeast--a review.Lipids. 1995 Mar;30(3):227-30. doi: 10.1007/BF02537825. Lipids. 1995. PMID: 7791530 Review.
-
Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae.Genes (Basel). 2020 Jul 15;11(7):795. doi: 10.3390/genes11070795. Genes (Basel). 2020. PMID: 32679672 Free PMC article. Review.
Cited by
-
Inhibition of cell cycle-dependent hyphal and biofilm formation by a novel cytochalasin 19,20‑epoxycytochalasin Q in Candida albicans.Sci Rep. 2023 Jun 15;13(1):9724. doi: 10.1038/s41598-023-36191-4. Sci Rep. 2023. PMID: 37322086 Free PMC article.
-
Media optimization of antimicrobial activity production and beta-glucan content of endophytic fungi Xylaria sp. BCC 1067.Biotechnol Rep (Amst). 2022 May 27;35:e00742. doi: 10.1016/j.btre.2022.e00742. eCollection 2022 Sep. Biotechnol Rep (Amst). 2022. PMID: 35677324 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

