The benzylisoquinoline alkaloids, berberine and coptisine, act against camptothecin-resistant topoisomerase I mutants
- PMID: 33833336
- PMCID: PMC8032691
- DOI: 10.1038/s41598-021-87344-2
The benzylisoquinoline alkaloids, berberine and coptisine, act against camptothecin-resistant topoisomerase I mutants
Abstract
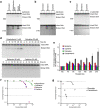
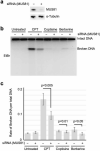
DNA replication inhibitors are utilized extensively in studies of molecular biology and as chemotherapy agents in clinical settings. The inhibition of DNA replication often triggers double-stranded DNA breaks (DSBs) at stalled DNA replication sites, resulting in cytotoxicity. In East Asia, some traditional medicines are administered as anticancer drugs, although the mechanisms underlying their pharmacological effects are not entirely understood. In this study, we screened Japanese herbal medicines and identified two benzylisoquinoline alkaloids (BIAs), berberine and coptisine. These alkaloids mildly induced DSBs, and this effect was dependent on the function of topoisomerase I (Topo I) and MUS81-EME1 structure-specific endonuclease. Biochemical analysis revealed that the action of BIAs involves inhibiting the catalytic activity of Topo I rather than inducing the accumulation of the Topo I-DNA complex, which is different from the action of camptothecin (CPT). Furthermore, the results showed that BIAs can act as inhibitors of Topo I, even against CPT-resistant mutants, and that the action of these BIAs was independent of CPT. These results suggest that using a combination of BIAs and CPT might increase their efficiency in eliminating cancer cells.
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
CK2-mediated hyperphosphorylation of topoisomerase I targets serine 506, enhances topoisomerase I-DNA binding, and increases cellular camptothecin sensitivity.PLoS One. 2012;7(11):e50427. doi: 10.1371/journal.pone.0050427. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185622 Free PMC article.
-
Mus81-mediated DNA cleavage resolves replication forks stalled by topoisomerase I-DNA complexes.J Cell Biol. 2011 Nov 28;195(5):739-49. doi: 10.1083/jcb.201104003. J Cell Biol. 2011. PMID: 22123861 Free PMC article.
-
New Topoisomerase I mutations are associated with resistance to camptothecin.Mol Cancer. 2011 May 27;10:64. doi: 10.1186/1476-4598-10-64. Mol Cancer. 2011. PMID: 21619602 Free PMC article.
-
Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors.Biochim Biophys Acta. 2013 Jan;1835(1):11-27. doi: 10.1016/j.bbcan.2012.09.002. Epub 2012 Sep 21. Biochim Biophys Acta. 2013. PMID: 23006513 Review.
-
[DNA topoisomerases targeting anticancer agents and mechanism for acquirement of drug resistance].Nihon Rinsho. 1997 May;55(5):1096-102. Nihon Rinsho. 1997. PMID: 9155159 Review. Japanese.
Cited by
-
Synthesis and Evaluation of (Bis)benzyltetrahydroisoquinoline Alkaloids as Antiparasitic Agents.JACS Au. 2024 Feb 12;4(2):847-854. doi: 10.1021/jacsau.4c00007. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425909 Free PMC article.
-
Isoquinoline Alkaloids from Coptis chinensis Franch: Focus on Coptisine as a Potential Therapeutic Candidate against Gastric Cancer Cells.Int J Mol Sci. 2022 Sep 7;23(18):10330. doi: 10.3390/ijms231810330. Int J Mol Sci. 2022. PMID: 36142236 Free PMC article.
-
Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents.Molecules. 2021 Aug 6;26(16):4778. doi: 10.3390/molecules26164778. Molecules. 2021. PMID: 34443363 Free PMC article.
-
Herbal Therapies for Cancer Treatment: A Review of Phytotherapeutic Efficacy.Biologics. 2024 Sep 10;18:229-255. doi: 10.2147/BTT.S484068. eCollection 2024. Biologics. 2024. PMID: 39281032 Free PMC article. Review.
-
Mechanism of action of non-camptothecin inhibitor Genz-644282 in topoisomerase I inhibition.Commun Biol. 2022 Sep 16;5(1):982. doi: 10.1038/s42003-022-03920-w. Commun Biol. 2022. PMID: 36114357 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

