Intranasal versus intratracheal exposure to lipopolysaccharides in a murine model of acute respiratory distress syndrome
- PMID: 33833346
- PMCID: PMC8032690
- DOI: 10.1038/s41598-021-87462-x
Intranasal versus intratracheal exposure to lipopolysaccharides in a murine model of acute respiratory distress syndrome
Abstract
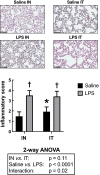
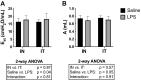
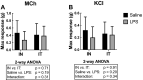
Due to frequent and often severe lung affections caused by COVID-19, murine models of acute respiratory distress syndrome (ARDS) are increasingly used in experimental lung research. The one induced by a single lipopolysaccharide (LPS) exposure is practical. However, whether it is preferable to administer LPS intranasally or intratracheally remains an open question. Herein, female C57Bl/6 J mice were exposed intranasally or intratracheally to one dose of either saline or 3 mg/kg of LPS. They were studied 24 h later. The groups treated with LPS, either intranasally or intratracheally, exhibited a pronounced neutrophilic inflammation, signs of lung tissue damage and protein extravasation into the alveoli, and mild lung dysfunction. The magnitude of the response was generally not different between groups exposed intranasally versus intratracheally. However, the variability of some the responses was smaller in the LPS-treated groups exposed intranasally versus intratracheally. Notably, the saline-treated mice exposed intratracheally demonstrated a mild neutrophilic inflammation and alterations of the airway epithelium. We conclude that an intranasal exposure is as effective as an intratracheal exposure in a murine model of ARDS induced by LPS. Additionally, the groups exposed intranasally demonstrated less variability in the responses to LPS and less complications associated with the sham procedure.
Conflict of interest statement
LF, DB and AR are employed by SCIREQ Inc., a commercial entity with interests in topics related to the content of the present work. DB also hold stocks. SCIREQ Inc. is an emka TECHNOLOGIES company. One device from SCIREQ Inc was used in the present study, namely the flexiVent. For any investigator who would like to use the methods described in the present study in the future, the flexiVent would only be required to report the outcomes of respiratory mechanics. None of SCIREQ employees or executives had decision rights over the manuscript prior to its submission for publication. FK, ASF, STP, ADM, CH, MBo, MBe, MM and YB have no conflict of interest.
Figures




References
-
- Global death from COVID-19. COVID-19 Map - John Hopkins Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

