Improvement in Lung Function and Patient-Reported Outcomes in Patients with COPD with Comorbid Anxiety and Depression Receiving Nebulized Glycopyrrolate in the GOLDEN 3 and 4 Studies
- PMID: 33833507
- PMCID: PMC8020329
- DOI: 10.2147/COPD.S294053
Improvement in Lung Function and Patient-Reported Outcomes in Patients with COPD with Comorbid Anxiety and Depression Receiving Nebulized Glycopyrrolate in the GOLDEN 3 and 4 Studies
Abstract
Background: Anxiety and depression (A/D) are common in patients with chronic obstructive pulmonary disease (COPD) and are often associated with lower adherence to treatment and worse patient-related outcomes. However, studies on the impact of comorbid A/D on responses to bronchodilators are limited.
Methods: This post hoc analysis of pooled data (N=861) from the GOLDEN 3 and 4 studies compared the efficacy and safety of nebulized glycopyrrolate (GLY) 25 µg in patients with moderate-to-very-severe COPD, grouped by self-reported A/D. Changes in forced expiratory volume in 1 second (FEV1) and health-related quality of life determined by St George's Respiratory Questionnaire (SGRQ) scores in patients with or without comorbid A/D (A/D [+] or A/D [-]) were examined following 12 weeks of GLY 25 µg twice-daily (BID) or placebo treatment.
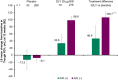
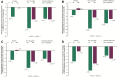
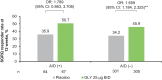
Results: A/D (+) patients were predominantly female, younger, included a higher proportion of current smokers, and had higher baseline SGRQ scores compared with the A/D (-) group. At 12 weeks, GLY resulted in placebo-adjusted improvements from baseline in FEV1 of 46.9 mL (p=0.19; not significant) and 106.7 mL (p<0.0001), in the A/D (+) and A/D (-) groups, respectively. Improvements were observed with GLY compared to placebo in SGRQ scores, regardless of baseline A/D status; the placebo-adjusted least squares mean change from baseline in SGRQ total scores was -3.16 (p>0.05) and -3.34 (p<0.001), for the A/D (+) and A/D (-) groups, respectively. Despite numerical improvements in SGRQ scores with GLY in the A/D (+) group, a higher response to placebo was observed. GLY was generally well tolerated throughout 12 weeks of treatment; incidence of adverse events was higher in the A/D (+) group compared with the A/D (-) group in both treatment arms.
Conclusion: GLY 25 µg BID resulted in numerical improvements in FEV1, SGRQ total scores and SGRQ responder rates in patients with moderate-to-very-severe COPD, regardless of A/D status at baseline; significant improvements were noted only in the A/D (+) group. The results emphasize the importance of considering underlying comorbidities including A/D when evaluating the efficacy of COPD treatments.
Keywords: COPD; LAMA; anxiety; depression; nebulized glycopyrrolate.
© 2021 Hanania et al.
Conflict of interest statement
NAH received honoraria for serving as a consultant or advisory boards for GlaxoSmithKline, Sanofi, Regeneron, Genentech, Novartis, Boehringer Ingelheim, Astra Zeneca, Teva, Amgen, and Mylan Pharmaceuticals. His institution receives research grant support from GlaxoSmithKline, Sanofi, Genentech, Gossamer Bio, Boehringer Ingelheim, Novartis, and AstraZeneca. AMY received an honorarium for consultation fees from AstraZeneca. AOG was an employee of Sunovion Pharmaceuticals Inc. at the time of the study and is currently an employee of Alexion Pharmaceuticals. MT, TG, SSh, and SSa are employees of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Impact of baseline clinical features on outcomes of nebulized glycopyrrolate therapy in COPD.NPJ Prim Care Respir Med. 2021 Oct 7;31(1):43. doi: 10.1038/s41533-021-00255-7. NPJ Prim Care Respir Med. 2021. PMID: 34620878 Free PMC article. Review.
-
Effect of SGRQ-Defined Chronic Bronchitis at Baseline on Treatment Outcomes in Patients with COPD Receiving Nebulized Glycopyrrolate.Int J Chron Obstruct Pulmon Dis. 2021 Apr 12;16:945-955. doi: 10.2147/COPD.S304182. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33880019 Free PMC article.
-
Effect of Gender on Lung Function and Patient-Reported Outcomes in Patients with COPD Receiving Nebulized Glycopyrrolate.Int J Chron Obstruct Pulmon Dis. 2020 May 6;15:995-1004. doi: 10.2147/COPD.S240303. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32440111 Free PMC article.
-
Efficacy and safety of a novel, nebulized glycopyrrolate for the treatment of COPD: effect of baseline disease severity and age; pooled analysis of GOLDEN 3 and GOLDEN 4.Int J Chron Obstruct Pulmon Dis. 2018 Dec 18;14:27-37. doi: 10.2147/COPD.S184808. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30587959 Free PMC article.
-
Correlations between FEV1 and patient-reported outcomes: A pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease.Pulm Pharmacol Ther. 2018 Apr;49:11-19. doi: 10.1016/j.pupt.2017.12.005. Epub 2017 Dec 19. Pulm Pharmacol Ther. 2018. PMID: 29277690 Review.
Cited by
-
Post Hoc Analysis of Lung Function Improvement and Patient-Reported Outcomes With Revefenacin in Adults With Moderate-to-Very Severe COPD and Comorbid Anxiety or Depression.Chronic Obstr Pulm Dis. 2024 Mar 26;11(2):196-205. doi: 10.15326/jcopdf.2023.0465. Chronic Obstr Pulm Dis. 2024. PMID: 38241514 Free PMC article.
-
Impact of baseline clinical features on outcomes of nebulized glycopyrrolate therapy in COPD.NPJ Prim Care Respir Med. 2021 Oct 7;31(1):43. doi: 10.1038/s41533-021-00255-7. NPJ Prim Care Respir Med. 2021. PMID: 34620878 Free PMC article. Review.
-
Impact of COVID-19, cancer survivorship and patient-provider communication on mental health in the US Difference-In-Difference.Npj Ment Health Res. 2023 Aug 30;2(1):14. doi: 10.1038/s44184-023-00034-x. Npj Ment Health Res. 2023. PMID: 38609572 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. Available from: http://goldcopd.org/. Accessed May20, 2019.
-
- Yohannes AM, Kaplan A, Hanania NA. COPD in primary care: key considerations for optimized management: anxiety and depression in chronic obstructive pulmonary disease: recognition and management. J Fam Pract. 2018;67(2 Suppl):S11–S18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

