Mutation Types of CYP71P1 Cause Different Phenotypes of Mosaic Spot Lesion and Premature Leaf Senescence in Rice
- PMID: 33833770
- PMCID: PMC8021961
- DOI: 10.3389/fpls.2021.641300
Mutation Types of CYP71P1 Cause Different Phenotypes of Mosaic Spot Lesion and Premature Leaf Senescence in Rice
Abstract
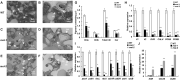
Lesion mimic mutants (LMMs) are ideal materials for studying programmed cell death and defense response in plants. Here we report investigations on two LMMs (msl-1 and msl-2) from the indica rice cultivar JG30 treated by ethyl methyl sulfone. Both of the mutants showed similar mosaic spot lesions at seedling stage, but they displayed different phenotypes along with development of the plants. At tillering stage, larger orange spots appeared on leaves of msl-2, while only small reddish-brown spots exhibit on leaves of msl-1. At heading stage, the msl-2 plants were completely dead, while the msl-1 plants were still alive even if showed apparent premature senility. For both the mutants, the mosaic spot lesion formation was induced by light; DAB and trypan blue staining showed a large amount of hydrogen peroxide accumulated at the lesion sites, accompanied by a large number of cell death. Consequently, reactive oxygen species were enriched in leaves of the mutants; SOD and CAT activities in the scavenging enzyme system were decreased compared with the wild type. In addition, degraded chloroplasts, decreased photosynthetic pigment content, down-regulated expression of genes associated with chloroplast synthesis/photosynthesis and up-regulated expression of genes related to senescence were detected in the mutants, but the abnormality of msl-2 was more serious than that of msl-1 in general. Genetic analysis and map-based cloning revealed that the lesion mimic and premature senescence traits of both the mutants were controlled by recessive mutated alleles of the SL (Sekiguchi lesion) gene, which encodes the CYP71P1 protein belonging to cytochrome P450 monooxygenase family. The difference of mutation sites and mutation types (SNP-caused single amino acid change and SNP-caused early termination of translation) led to the different phenotypes in severity between msl-1 and msl-2. Taken together, this work revealed that the CYP71P1 is involved in regulation of both premature senescence and cell death in rice, and its different mutation sites and mutation types could cause different phenotypes in terms of severity.
Keywords: rice; cell death; leaf senescence; lesion mimic mutants; mutation types; reactive oxygen species.
Copyright © 2021 Zheng, Xu, Wang, Tang, Wei, Ji, Wang and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Lesion Mimic Mutant: An Ideal Genetic Material for Deciphering the Balance Between Plant Immunity and Growth.Rice (N Y). 2025 May 13;18(1):34. doi: 10.1186/s12284-025-00789-1. Rice (N Y). 2025. PMID: 40355767 Free PMC article. Review.
-
Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza sativa L.).Int J Mol Sci. 2018 Jan 3;19(1):140. doi: 10.3390/ijms19010140. Int J Mol Sci. 2018. PMID: 29301377 Free PMC article.
-
Characterization and fine mapping of a light-dependent leaf lesion mimic mutant 1 in rice.Plant Physiol Biochem. 2015 Dec;97:44-51. doi: 10.1016/j.plaphy.2015.09.001. Epub 2015 Sep 12. Plant Physiol Biochem. 2015. PMID: 26410574
-
Mutation of rice EARLY LEAF LESION AND SENESCENCE 1 (ELS1), which encodes an anthranilate synthase α-subunit, induces ROS accumulation and cell death through activating the tryptophan synthesis pathway in rice.Plant J. 2024 Dec;120(6):2723-2737. doi: 10.1111/tpj.17141. Epub 2024 Nov 14. Plant J. 2024. PMID: 39540877
-
Rice Lesion Mimic Mutants (LMM): The Current Understanding of Genetic Mutations in the Failure of ROS Scavenging during Lesion Formation.Plants (Basel). 2021 Aug 4;10(8):1598. doi: 10.3390/plants10081598. Plants (Basel). 2021. PMID: 34451643 Free PMC article. Review.
Cited by
-
BrCYP71 mutation resulted in stay-green in pak choi (Brassica rapa L. ssp. chinensis).Theor Appl Genet. 2025 Jan 28;138(2):37. doi: 10.1007/s00122-025-04829-8. Theor Appl Genet. 2025. PMID: 39875710
-
Comparative Proteomic Analysis Reveals the Ascorbate Peroxidase-Mediated Plant Resistance to Verticillium dahliae in Gossypium barbadense.Front Plant Sci. 2022 May 19;13:877146. doi: 10.3389/fpls.2022.877146. eCollection 2022. Front Plant Sci. 2022. PMID: 35665163 Free PMC article.
-
A conserved clathrin-coated vesicle component, OsSCYL2, regulates plant innate immunity in rice.Plant Cell Environ. 2022 Feb;45(2):542-555. doi: 10.1111/pce.14240. Epub 2021 Dec 21. Plant Cell Environ. 2022. PMID: 34866195 Free PMC article.
-
Lesion Mimic Mutant: An Ideal Genetic Material for Deciphering the Balance Between Plant Immunity and Growth.Rice (N Y). 2025 May 13;18(1):34. doi: 10.1186/s12284-025-00789-1. Rice (N Y). 2025. PMID: 40355767 Free PMC article. Review.
-
Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis.Plants (Basel). 2025 Apr 1;14(7):1072. doi: 10.3390/plants14071072. Plants (Basel). 2025. PMID: 40219140 Free PMC article.
References
-
- Arase S., Ueno M., Toko M., Honda Y., Itoh K., Ozoe Y. (2001). Light-dependent accumulation of tryptamine in the rice sekiguchi lesion mutant infected with Magnaporthe grisea. J. Phytopathol. 149 409–413. 10.1046/j.1439-0434.2001.00646.x - DOI
-
- Buchanan-Wollaston V. (1997). The molecular biology of leaf senescence. J. Exp. Bot. 2 181–199.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

