Resveratrol Derivative, Trans-3, 5, 4'-Trimethoxystilbene Sensitizes Osteosarcoma Cells to Apoptosis via ROS-Induced Caspases Activation
- PMID: 33833855
- PMCID: PMC8018847
- DOI: 10.1155/2021/8840692
Resveratrol Derivative, Trans-3, 5, 4'-Trimethoxystilbene Sensitizes Osteosarcoma Cells to Apoptosis via ROS-Induced Caspases Activation
Abstract
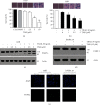
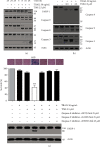
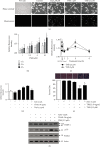
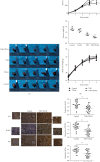
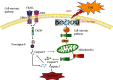
Numerous studies have shown that resveratrol can induce apoptosis in cancer cells. Trans-3, 5, 4'-trimethoxystilbene (TMS), a novel derivative of resveratrol, is a more potent anticancer compound than resveratrol and can induce apoptosis in cancer cells. Herein, we examined the mechanisms involved in TMS-mediated sensitization of human osteosarcoma (143B) cells to TNF-related apoptosis-inducing ligand- (TRAIL-) induced apoptosis. Our results showed that cotreatment with TSM and TRAIL activated caspases and increased PARP-1 cleavage in 143B cells. Decreasing cellular ROS levels using NAC reversed TSM- and TRAIL-induced apoptosis in 143B cells. NAC abolished the upregulated expression of PUMA and p53 induced by treatment with TRAIL and TSM. Silencing the expression of p53 or PUMA using RNA interference attenuated TSM-mediated sensitization of 143B cells to TRAIL-induced apoptosis. Knockdown of Bax also reversed TSM-induced sensitization of 143B cell to TRAIL-mediated apoptotic cell death. These results indicate that cotreatment with TRAIL and TSM evaluated intracellular ROS level, promoted DNA damage, and activated the Bax/PUMA/p53 pathway, leading to activation of both mitochondrial and caspase-mediated apoptosis in 143B cells. Orthotopic implantation of 143B cells in mice also demonstrated that cotreatment with TRAIL and TSM reversed resistance to apoptosis in cells without obvious adverse effects in normal cells.
Copyright © 2021 Yu Feng et al.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures





References
-
- Yang C. C., Hsiao L. D., Lin H. H., et al. Induction of HO-1 by 5, 8-Dihydroxy-4,7-Dimethoxyflavone via Activation of ROS/p38 MAPK/Nrf2 Attenuates Thrombin-Induced Connective Tissue Growth Factor Expression in Human Cardiac Fibroblasts. Oxidative Medicine and Cellular Longevity. 2020;2020:18. doi: 10.1155/2020/1080168.1080168 - DOI - PMC - PubMed
-
- Hong M., Li J., Li S., Almutairi M. M. Resveratrol Derivative,Trans‐3, 5, 4’‐Trimethoxystilbene, prevents the developing of atherosclerotic lesions and attenuates cholesterol accumulation in macrophage foam cells. Molecular Nutrition & Food Research. 2020;64(6, article e1901115) doi: 10.1002/mnfr.201901115. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

