Antioxidant, Cytotoxicity and Cytoprotective Potential of Extracts of Grewia Flava and Grewia Bicolor Berries
- PMID: 33833897
- PMCID: PMC8010423
- DOI: 10.3831/KPI.2021.24.1.24
Antioxidant, Cytotoxicity and Cytoprotective Potential of Extracts of Grewia Flava and Grewia Bicolor Berries
Abstract
Objectives: Accumulation of cellular reactive oxygen species (ROS) leads to oxidative stress. Increased production of ROS, such as superoxide anion, or a deficiency in their clearance by antioxidant defences, mediates cellular pathology. Grewia Spp fruits are a source of bioactive compounds and have notable antioxidant activity. Although the antioxidant capacity of Grewia Spp has been studied, there is very limited evidence that links the antioxidant activities of Grewia bicolor and Grewia flava to the inhibition of free radical formation associated with damage in biological systems.
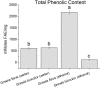
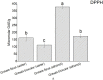
Methods: This study evaluated the protective effects of Grewia bicolor and Grewia flava extracts against free radical-induced oxidative stress and the resulting cytotoxicity effect using HeLa cells. Antioxidant properties determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and total phenolic content (TPC) assays showed significantly higher (p < 0.05) antioxidant activity in Grewia flava (ethanol extract) than Grewia flava (water extract) and Grewia bicolor (ethanol and water extracts).
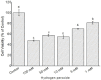
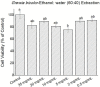
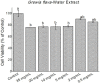
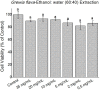
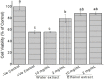
Results: Using 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide or MTT assay, cytotoxicity results showed that extracts of Grewia bicolor and Grewia flava were less toxic to HeLa cells at tested concentrations compared to the untreated control. This confirmed the low toxicity of these edible fruits at the tested concentrations in HeLa cells. Furthermore, hydrogen peroxide (H2O2)-induced cell loss was effectively reduced by pre-incubating HeLa cells with Grewia bicolor and Grewia flava extracts, with Grewia flava (ethanol extract) revealing better protection.
Conclusion: The effect was speculated to be associated with the higher antioxidant activity of Grewia flava (ethanol extract). Additional studies will warrant confirmation of the mechanism of action of such effects.
Keywords: antioxidant; cytoprotective; cytotoxicity; grewia bicolor; grewia flava; reactive oxygen species.
© 2021 Korean Pharmacopuncture Institute.
Conflict of interest statement
CONFLICTS OF INTEREST All authors declare no conflict of interest.
Figures


References
-
- Hayakawa M, Kuzuya F. [Free radicals and diabetes mellitus] Nihon Ronen Igakkai Zasshi. 1990;27(2):149–54. Japanese. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
