Performance of International AIDS Vaccine Initiative African clinical research laboratories in standardised ELISpot and peripheral blood mononuclear cell processing in support of HIV vaccine clinical trials
- PMID: 33833946
- PMCID: PMC8014752
- DOI: 10.4102/ajlm.v10i1.1056
Performance of International AIDS Vaccine Initiative African clinical research laboratories in standardised ELISpot and peripheral blood mononuclear cell processing in support of HIV vaccine clinical trials
Abstract
Background: Standardisation of procedures for performing cellular functional assays across laboratories participating in multicentre clinical trials is key for generating comparable and reliable data.
Objective: This article describes the performance of accredited laboratories in Africa and Europe on testing done in support of clinical trials.
Methods: For enzyme-linked immunospot assay (ELISpot) proficiency, characterised peripheral blood mononuclear cells (PBMCs) obtained from 48 HIV-negative blood donors in Johannesburg, South Africa, were sent to participating laboratories between February 2010 and February 2014. The PBMCs were tested for responses against cytomegalovirus, Epstein Barr and influenza peptide pools in a total of 1751 assays. In a separate study, a total of 1297 PBMC samples isolated from healthy HIV-negative participants in clinical trials of two prophylactic HIV vaccine candidates in Kenya, Uganda, Rwanda and Zambia were analysed for cell viability, cell yield and cell recovery from frozen PBMCs.
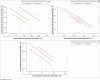
Results: Most (99%) of the 1751 ELISpot proficiency assays had data within acceptable ranges with low responses to mock stimuli. No significant statistical difference were observed in ELISpot responses at the five laboratories actively conducting immunological analyses. Of the 1297 clinical trial PBMCs processed, 94% had cell viability above 90% and 96% had cell yield above 0.7 million per mL of blood in freshly isolated cells. All parameters were within the predefined acceptance criteria.
Conclusion: We demonstrate that multiple laboratories can generate reliable, accurate and comparable data by using standardised procedures, having regular training, having regular equipment maintenance and using centrally sourced reagents.
Keywords: ELISpot; PBMC processing; clinical trials; good clinical laboratory practice; peripheral blood mononuclear cells; proficiency testing.
© 2021. The Authors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
