Dihydrocapsaicin Inhibits Cell Proliferation and Metastasis in Melanoma via Down-regulating β-Catenin Pathway
- PMID: 33833997
- PMCID: PMC8023049
- DOI: 10.3389/fonc.2021.648052
Dihydrocapsaicin Inhibits Cell Proliferation and Metastasis in Melanoma via Down-regulating β-Catenin Pathway
Erratum in
-
Corrigendum: Dihydrocapsaicin inhibits cell proliferation and metastasis in melanoma via down-regulating β-catenin pathway.Front Oncol. 2022 Oct 11;12:1052365. doi: 10.3389/fonc.2022.1052365. eCollection 2022. Front Oncol. 2022. PMID: 36303842 Free PMC article.
Abstract
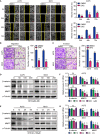
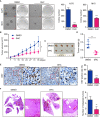
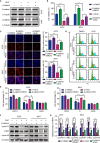
Dihydrocapsaicin (DHC) is one of the main components of capsaicinoids in Capsicum. It has been reported that DHC exerts anti-cancer effects on diverse malignant tumors, such as colorectal cancer, breast cancer, and glioma. However, studies focused on the effect of DHC upon melanoma have rarely been done. In the present study, melanoma A375 and MV3 cell lines were treated with DHC and the cell proliferation, migration, and invasion were significantly suppressed. Furthermore, DHC effectively inhibited xenograft tumor growth and pulmonary metastasis of melanoma cells in NOD/SCID mice model. It was identified that β-catenin, which plays significant roles in cell proliferation and epithelial-mesenchymal transition, was down-regulated after DHC treatment. In addition, cyclin D1, c-Myc, MMP2, and MMP7, which are critical in diverse cellular process regulation as downstream proteins of β-catenin, were all decreased. Mechanistically, DHC accelerates ubiquitination of β-catenin and up-regulates the beta-transducin repeat containing E3 ubiquitin protein ligase (BTRC) in melanoma cells. The DHC induced suppression of cell proliferation, migration, and invasion were partly rescued by exogenous β-catenin overexpression, both in vitro and in vivo. Taken together, DHC may serve as a candidate natural compound for human melanoma treatment through β-catenin pathway.
Keywords: cell proliferation; dihydrocapsaicin; melanoma; metastasis; ubiquitination; β-catenin.
Copyright © 2021 Shi, Li, Zhang, Deng, Liu, Du, Li, Ji, Guo, Liu, Hu, Liu and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Dehydrocorydaline inhibits cell proliferation, migration and invasion via suppressing MEK1/2-ERK1/2 cascade in melanoma.Onco Targets Ther. 2019 Jul 2;12:5163-5175. doi: 10.2147/OTT.S183558. eCollection 2019. Onco Targets Ther. 2019. PMID: 31456643 Free PMC article.
-
Cinobufacini Inhibits Colon Cancer Invasion and Metastasis via Suppressing Wnt/β-Catenin Signaling Pathway and EMT.Am J Chin Med. 2020;48(3):703-718. doi: 10.1142/S0192415X20500354. Epub 2020 Apr 24. Am J Chin Med. 2020. PMID: 32329642
-
ZNF750 inhibits the proliferation and invasion of melanoma cells through modulating the Wnt/b-catenin signaling pathway.Folia Histochem Cytobiol. 2020;58(4):255-263. doi: 10.5603/FHC.a2020.0026. Epub 2020 Nov 13. Folia Histochem Cytobiol. 2020. PMID: 33185885
-
Overexpression of FOXS1 in gastric cancer cell lines inhibits proliferation, metastasis, and epithelial-mesenchymal transition of tumor through downregulating wnt/β-catenin pathway.J Cell Biochem. 2019 Mar;120(3):2897-2907. doi: 10.1002/jcb.26821. Epub 2018 Nov 30. J Cell Biochem. 2019. PMID: 30500980
-
Alantolactone Inhibits Melanoma Cell Culture Viability and Migration and Promotes Apoptosis by Inhibiting Wnt/β-Catenin Signaling.Anticancer Agents Med Chem. 2023;23(1):94-104. doi: 10.2174/1871520622666220519100054. Anticancer Agents Med Chem. 2023. PMID: 35598249
Cited by
-
Dihydrocapsaicin Enhances Tumor Necrosis Factor-α-Induced Apoptosis and G1 Cell Cycle Arrest in Human Cervical Cancer Cells Through TAK1-Mediated NF-κB and EGFR Pathways.Int J Mol Sci. 2025 May 22;26(11):5011. doi: 10.3390/ijms26115011. Int J Mol Sci. 2025. PMID: 40507823 Free PMC article.
-
Morusin shows potent antitumor activity for melanoma through apoptosis induction and proliferation inhibition.BMC Cancer. 2023 Jun 29;23(1):602. doi: 10.1186/s12885-023-11080-1. BMC Cancer. 2023. PMID: 37386395 Free PMC article.
-
Blocking the WNT/β-catenin pathway in cancer treatment:pharmacological targets and drug therapeutic potential.Heliyon. 2024 Aug 12;10(16):e35989. doi: 10.1016/j.heliyon.2024.e35989. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253139 Free PMC article. Review.
-
Research Progress of Natural Compounds from Chinese Herbal Medicine in the Treatment of Melanoma.Curr Treat Options Oncol. 2025 Jul;26(7):533-568. doi: 10.1007/s11864-025-01322-8. Epub 2025 May 15. Curr Treat Options Oncol. 2025. PMID: 40372659 Review.
-
The effect and mechanism of selenium supplementation on the proliferation capacity of bovine endometrial epithelial cells exposed to lipopolysaccharide in vitro under high cortisol background.J Anim Sci. 2024 Jan 3;102:skae021. doi: 10.1093/jas/skae021. J Anim Sci. 2024. PMID: 38289713 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

