Antimicrobial Peptide-Polymer Conjugates for Dentistry
- PMID: 33834166
- PMCID: PMC8026165
- DOI: 10.1021/acsapm.9b00921
Antimicrobial Peptide-Polymer Conjugates for Dentistry
Abstract
Bacterial adhesion and growth at the composite/adhesive/tooth interface remain the primary cause of dental composite restoration failure. Early colonizers, including Streptococcus mutans, play a critical role in the formation of dental caries by creating an environment that reduces the adhesive's integrity. Subsequently, other bacterial species, biofilm formation, and lactic acid from S. mutans demineralize the adjoining tooth. Because of their broad spectrum of antibacterial activity and low risk for antibiotic resistance, antimicrobial peptides (AMPs) have received significant attention to prevent bacterial biofilms. Harnessing the potential of AMPs is still very limited in dentistry-a few studies have explored peptide-enabled antimicrobial adhesive copolymer systems using mainly nonspecific adsorption. In the current investigation, to avoid limitations from nonspecific adsorption and to prevent potential peptide leakage out of the resin, we conjugated an AMP with a commonly used monomer for dental adhesive formulation. To tailor the flexibility between the peptide and the resin material, we designed two different spacer domains. The spacer-integrated antimicrobial peptides were conjugated to methacrylate (MA), and the resulting MA-AMP monomers were next copolymerized into dental adhesives as AMP-polymer conjugates. The resulting bioactivity of the polymethacrylate-based AMP conjugated matrix activity was investigated. The antimicrobial peptide conjugated to the resin matrix demonstrated significant antimicrobial activity against S. mutans. Secondary structure analyses of conjugated peptides were applied to understand the activity differential. When mechanical properties of the adhesive system were investigated with respect to AMP and cross-linking concentration, resulting AMP-polymer conjugates maintained higher compressive moduli compared to hydrogel analogues including polyHEMA. Overall, our result provides a robust approach to develop a fine-tuned bioenabled peptide adhesive system with improved mechanical properties and antimicrobial activity. The results of this study represent a critical step toward the development of peptide-conjugated dentin adhesives for treatment of secondary caries and the enhanced durability of dental composite restorations.
Keywords: Streptococcus mutans; antimicrobial peptide; bioactivity; bioconjugation; dental adhesive; mechanical property.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
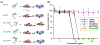



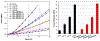
References
-
- Delaviz Y; Finer Y; Santerre JP Biodegradation of Resin Composites and Adhesives by Oral Bacteria and Saliva: A Rationale for New Material Designs that Consider the Clinical Environment and Treatment Challenges. Dent. Mater 2014, 30 (1), 16–32. - PubMed
-
- Moszner N; Hirt T New Polymer-Chemical Developments in Clinical Dental Polymer Materials: Enamel—Dentin Adhesives and Restorative Composites. J. Polym. Sci., Part A: Polym. Chem 2012, 50 (21), 4369–4402.
-
- Santerre J; Shajii L; Leung B Relation of Dental Composite Formulations to Their Degradation and the Release of Hydrolyzed Polymeric-Resin-Derived Products. Crit. Rev. Oral Biol. Med 2001, 12 (2), 136–151. - PubMed
-
- Rho YJ; Namgung C; Jin BH; Lim BS; Cho BH Longevity of Direct Restorations in Stress-Bearing Posterior Cavities: A Retrospective Study. Oper. Dent 2013, 38 (6), 572–82. - PubMed
-
- Bernardo M; Luis H; Martin MD; Leroux BG; Rue T; Leitao J; DeRouen TA Survival and Reasons for Failure of Amalgam versus Composite Posterior Restorations Placed in a Randomized Clinical Trial. J. Am. Dent. Assoc., JADA 2007, 138 (6), 775–783. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

