Remibrutinib (LOU064): A selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial
- PMID: 33834628
- PMCID: PMC8504815
- DOI: 10.1111/cts.13005
Remibrutinib (LOU064): A selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial
Abstract
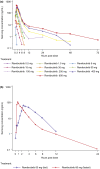
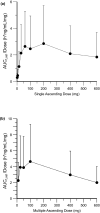
Safe and effective new oral therapies for autoimmune, allergic, and inflammatory conditions remain a significant therapeutic need. Here, we investigate the human pharmacokinetics, pharmacodynamics (PDs), and safety of the selective, covalent Bruton's tyrosine kinase (BTK) inhibitor, remibrutinib. Study objectives were explored in randomized single and multiple ascending dose (SAD and MAD, respectively) cohorts with daily doses up to 600 mg, and a crossover food effect (FE) cohort, in adult healthy subjects without (SAD [n =80]/FE [n =12]) or with asymptomatic atopic diathesis (MAD [n =64]). A single oral dose of remibrutinib (0.5-600 mg) was rapidly absorbed (time to maximum concentration = 0.5 h-1.25 h) with an apparent blood clearance of 280-560 L/h and apparent volume of distribution of 400-15,000 L. With multiple doses (q.d. and b.i.d.), no pronounced accumulation of remibrutinib was detected (mean residence time was <3 h). Food intake showed no clinically relevant effect on remibrutinib exposure suggesting no need for dose adaptation. With remibrutinib doses greater than or equal to 30 mg, blood BTK occupancy was greater than 95% for at least 24 h (SAD). With MAD, remibrutinib reached near complete blood BTK occupancy at day 12 predose with greater than or equal to 10 mg q.d. Near complete basophil or skin prick test (SPT) inhibition at day 12 predose was achieved at greater than or equal to 50 mg q.d. for CD63 and at greater than or equal to 100 mg q.d. for SPT. Remibrutinib was well-tolerated at all doses without any dose-limiting toxicity. Remibrutinib showed encouraging blood and skin PDs with a favorable safety profile, supporting further development for diseases driven by mast cells, basophils, and B-cells, such as chronic spontaneous urticaria, allergic asthma, or Sjögren's syndrome.
© 2021 Novartis Institutes for BioMedical Research. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.Ka., P.E., M.C., A.J., M.Ki., A.K., A.M., and B.C. are shareholders and employees of Novartis, the company which is developing remibrutinib as pharmacological treatment in relevant indications. C.S. is an employee of GCE Solutions, the Clinical Research Organization sponsored by Novartis for the analysis of the study data. A.S. and R.F. are employees of Parexel, the Clinical Research Organization sponsored by Novartis for the execution of this study.
Figures

References
-
- Liang C, Tian D, Ren X, et al. The development of Bruton's tyrosine kinase (BTK) inhibitors from 2012 to 2017: a mini‐review. Eur J Med Chem. 2018;151:315‐326. - PubMed
-
- Norman P. Investigational Bruton's tyrosine kinase inhibitors for the treatment of rheumatoid arthritis. Expert Opin Investig Drugs. 2016;25:891‐899. - PubMed
-
- Rawlings DJ, Witte ON. The Btk subfamily of cytoplasmic tyrosine kinases: structure, regulation and function. Semin Immunol. 1995;7:237‐246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

