Applying A/B Testing to Clinical Decision Support: Rapid Randomized Controlled Trials
- PMID: 33835035
- PMCID: PMC8065554
- DOI: 10.2196/16651
Applying A/B Testing to Clinical Decision Support: Rapid Randomized Controlled Trials
Abstract
Background: Clinical decision support (CDS) is a valuable feature of electronic health records (EHRs) designed to improve quality and safety. However, due to the complexities of system design and inconsistent results, CDS tools may inadvertently increase alert fatigue and contribute to physician burnout. A/B testing, or rapid-cycle randomized tests, is a useful method that can be applied to the EHR in order to rapidly understand and iteratively improve design choices embedded within CDS tools.
Objective: This paper describes how rapid randomized controlled trials (RCTs) embedded within EHRs can be used to quickly ascertain the superiority of potential CDS design changes to improve their usability, reduce alert fatigue, and promote quality of care.
Methods: A multistep process combining tools from user-centered design, A/B testing, and implementation science was used to understand, ideate, prototype, test, analyze, and improve each candidate CDS. CDS engagement metrics (alert views, acceptance rates) were used to evaluate which CDS version is superior.
Results: To demonstrate the impact of the process, 2 experiments are highlighted. First, after multiple rounds of usability testing, a revised CDS influenza alert was tested against usual care CDS in a rapid (~6 weeks) RCT. The new alert text resulted in minimal impact on reducing firings per patients per day, but this failure triggered another round of review that identified key technical improvements (ie, removal of dismissal button and firings in procedural areas) that led to a dramatic decrease in firings per patient per day (23.1 to 7.3). In the second experiment, the process was used to test 3 versions (financial, quality, regulatory) of text supporting tobacco cessation alerts as well as 3 supporting images. Based on 3 rounds of RCTs, there was no significant difference in acceptance rates based on the framing of the messages or addition of images.
Conclusions: These experiments support the potential for this new process to rapidly develop, deploy, and rigorously evaluate CDS within an EHR. We also identified important considerations in applying these methods. This approach may be an important tool for improving the impact of and experience with CDS.
Trial registration: Flu alert trial: ClinicalTrials.gov NCT03415425; https://clinicaltrials.gov/ct2/show/NCT03415425. Tobacco alert trial: ClinicalTrials.gov NCT03714191; https://clinicaltrials.gov/ct2/show/NCT03714191.
Keywords: AB testing; alert fatigue; clinical decision support; clinical informatics; randomized controlled trials; usability.
©Jonathan Austrian, Felicia Mendoza, Adam Szerencsy, Lucille Fenelon, Leora I Horwitz, Simon Jones, Masha Kuznetsova, Devin M Mann. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 09.04.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


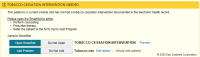





References
-
- Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of "corollary orders" to prevent errors of omission. J Am Med Inform Assoc. 1997;4(5):364–75. doi: 10.1136/jamia.1997.0040364. http://europepmc.org/abstract/MED/9292842 - DOI - PMC - PubMed
-
- Bernstein SL, Whitaker D, Winograd J, Brennan JA. An electronic chart prompt to decrease proprietary antibiotic prescription to self-pay patients. Acad Emerg Med. 2005 Mar;12(3):225–31. doi: 10.1197/j.aem.2004.09.021. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pu... - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

