Cyclically stretched ACL fibroblasts emigrating from spheroids adapt their cytoskeleton and ligament-related expression profile
- PMID: 33835257
- PMCID: PMC8211585
- DOI: 10.1007/s00441-021-03416-9
Cyclically stretched ACL fibroblasts emigrating from spheroids adapt their cytoskeleton and ligament-related expression profile
Erratum in
-
Correction to: Cyclically stretched ACL fibroblasts emigrating from spheroids adapt their cytoskeleton and ligament-related expression profile.Cell Tissue Res. 2021 Jun;384(3):789. doi: 10.1007/s00441-021-03472-1. Cell Tissue Res. 2021. PMID: 33977325 Free PMC article. No abstract available.
Abstract
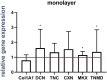
Mechanical stress of ligaments varies; hence, ligament fibroblasts must adapt their expression profile to novel mechanomilieus to ensure tissue resilience. Activation of the mechanoreceptors leads to a specific signal transduction, the so-called mechanotransduction. However, with regard to their natural three-dimensional (3D) microenvironment cell reaction to mechanical stimuli during emigrating from a 3D spheroid culture is still unclear. This study aims to provide a deeper understanding of the reaction profile of anterior cruciate ligament (ACL)-derived fibroblasts exposed to cyclic uniaxial strain in two-dimensional (2D) monolayer culture and during emigration from 3D spheroids with respect to cell survival, cell and cytoskeletal orientation, distribution, and expression profile. Monolayers and spheroids were cultured in crosslinked polydimethyl siloxane (PDMS) elastomeric chambers and uniaxially stretched (14% at 0.3 Hz) for 48 h. Cell vitality, their distribution, nuclear shape, stress fiber orientation, focal adhesions, proliferation, expression of ECM components such as sulfated glycosaminoglycans, collagen type I, decorin, tenascin C and cell-cell communication-related gap junctional connexin (CXN) 43, tendon-related markers Mohawk and tenomodulin (myodulin) were analyzed. In contrast to unstretched cells, stretched fibroblasts showed elongation of stress fibers, cell and cytoskeletal alignment perpendicular to strain direction, less rounded cell nuclei, increased numbers of focal adhesions, proliferation, amplified CXN43, and main ECM component expression in both cultures. The applied cyclic stretch protocol evoked an anabolic response and enhanced tendon-related marker expression in ACL-derived fibroblasts emigrating from 3D spheroids and seems also promising to support in future tissue formation in ACL scaffolds seeded in vitro with spheroids.
Keywords: ACL-derived fibroblasts; Connexin 43; Cyclic strain; Mechanostimulation; Mohawk; Myodulin; Spheroids; Tendon extracellular matrix; Uniaxial stretch.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




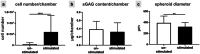

Similar articles
-
Maintenance of Ligament Homeostasis of Spheroid-Colonized Embroidered and Functionalized Scaffolds after 3D Stretch.Int J Mol Sci. 2021 Jul 30;22(15):8204. doi: 10.3390/ijms22158204. Int J Mol Sci. 2021. PMID: 34360970 Free PMC article.
-
Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-lactide-co-ε-caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams.Int J Mol Sci. 2020 Feb 8;21(3):1132. doi: 10.3390/ijms21031132. Int J Mol Sci. 2020. PMID: 32046263 Free PMC article.
-
Canine ACL fibroblast integrin expression and cell alignment in response to cyclic tensile strain in three-dimensional collagen gels.J Orthop Res. 2006 Mar;24(3):481-90. doi: 10.1002/jor.20050. J Orthop Res. 2006. PMID: 16453340
-
How do fibroblasts translate mechanical signals into changes in extracellular matrix production?Matrix Biol. 2003 Mar;22(1):73-80. doi: 10.1016/s0945-053x(03)00004-0. Matrix Biol. 2003. PMID: 12714044 Review.
-
Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies.Arthroscopy. 2006 Apr;22(4):441-51. doi: 10.1016/j.arthro.2006.01.017. Arthroscopy. 2006. PMID: 16581458 Review.
Cited by
-
Minispheroids as a Tool for Ligament Tissue Engineering: Do the Self-Assembly Techniques and Spheroid Dimensions Influence the Cruciate Ligamentocyte Phenotype?Int J Mol Sci. 2021 Oct 12;22(20):11011. doi: 10.3390/ijms222011011. Int J Mol Sci. 2021. PMID: 34681672 Free PMC article.
-
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology.Cells Tissues Organs. 2025;214(2):128-147. doi: 10.1159/000541416. Epub 2024 Sep 12. Cells Tissues Organs. 2025. PMID: 39265553 Free PMC article. Review.
-
Integrating Modern Technologies into Traditional Anterior Cruciate Ligament Tissue Engineering.Bioengineering (Basel). 2025 Jan 7;12(1):39. doi: 10.3390/bioengineering12010039. Bioengineering (Basel). 2025. PMID: 39851313 Free PMC article. Review.
References
-
- Abiko H, Fujiwara S, Ohashi K, Hiatari R, Mashiko T, Sakamoto N, Sato M, Mizuno K. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J Cell Sci. 2015;128:1683–1695. - PubMed
-
- Ahmed WW, Wolfram T, Goldyn AM, Bruellhoff K, Rioja BA, Moller M, Spatz JP, Saif TA, Groll J, Kemkemer R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials. 2010;31:250–258. - PubMed
-
- Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

