IL-10 lentivirus-laden hydrogel tubes increase spinal progenitor survival and neuronal differentiation after spinal cord injury
- PMID: 33835500
- PMCID: PMC9580015
- DOI: 10.1002/bit.27781
IL-10 lentivirus-laden hydrogel tubes increase spinal progenitor survival and neuronal differentiation after spinal cord injury
Abstract
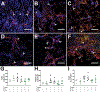
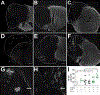
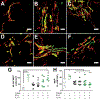
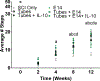
A complex cellular cascade characterizes the pathophysiological response following spinal cord injury (SCI) limiting regeneration. Biomaterial and stem cell combination therapies together have shown synergistic effects, compared to the independent benefits of each intervention, and represent a promising approach towards regaining function after injury. In this study, we combine our polyethylene glycol (PEG) cell delivery platform with lentiviral-mediated overexpression of the anti-inflammatory cytokine interleukin (IL)-10 to improve mouse embryonic Day 14 (E14) spinal progenitor transplant survival. Immediately following injury in a mouse SCI hemisection model, five PEG tubes were implanted followed by direct injection into the tubes of lentivirus encoding for IL-10. Two weeks after tube implantation, mouse E14 spinal progenitors were injected directly into the integrated tubes, which served as a soft substrate for cell transplantation. Together, the tubes with the IL-10 encoding lentivirus improved E14 spinal progenitor survival, assessed at 2 weeks posttransplantation (4 weeks postinjury). On average, 8.1% of E14 spinal progenitors survived in mice receiving IL-10 lentivirus-laden tubes compared with 0.7% in mice receiving transplants without tubes, an 11.5-fold difference. Surviving E14 spinal progenitors gave rise to neurons when injected into tubes. Axon elongation and remyelination were observed, in addition to a significant increase in functional recovery in mice receiving IL-10 lentivirus-laden tubes with E14 spinal progenitor delivery compared to the injury only control by 4 weeks postinjury. All other conditions did not exhibit increased stepping until 8 or 12 weeks postinjury. This system affords increased control over the transplantation microenvironment, offering the potential to improve stem cell-mediated tissue regeneration.
Keywords: biomaterials; gene delivery; neural stem cells; spinal cord injury; tissue engineering.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Figures
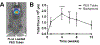

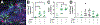
Similar articles
-
Acute Implantation of Aligned Hydrogel Tubes Supports Delayed Spinal Progenitor Implantation.ACS Biomater Sci Eng. 2020 Oct 12;6(10):5771-5784. doi: 10.1021/acsbiomaterials.0c00844. Epub 2020 Sep 14. ACS Biomater Sci Eng. 2020. PMID: 33320551 Free PMC article.
-
Spinal Progenitor-Laden Bridges Support Earlier Axon Regeneration Following Spinal Cord Injury.Tissue Eng Part A. 2018 Nov;24(21-22):1588-1602. doi: 10.1089/ten.TEA.2018.0053. Epub 2018 Oct 19. Tissue Eng Part A. 2018. PMID: 30215293 Free PMC article.
-
Sponge-mediated lentivirus delivery to acute and chronic spinal cord injuries.J Control Release. 2015 Apr 28;204:1-10. doi: 10.1016/j.jconrel.2015.02.032. Epub 2015 Feb 24. J Control Release. 2015. PMID: 25724274 Free PMC article.
-
Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury.Stem Cell Res Ther. 2021 Jan 7;12(1):36. doi: 10.1186/s13287-020-02090-y. Stem Cell Res Ther. 2021. PMID: 33413653 Free PMC article. Review.
-
Direct neuronal differentiation of neural stem cells for spinal cord injury repair.Stem Cells. 2021 Aug;39(8):1025-1032. doi: 10.1002/stem.3366. Epub 2021 Mar 5. Stem Cells. 2021. PMID: 33657255 Review.
Cited by
-
Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury.Cells. 2021 Aug 16;10(8):2102. doi: 10.3390/cells10082102. Cells. 2021. PMID: 34440872 Free PMC article. Review.
-
Human Amniotic Epithelial Stem Cells Promote Functional Recovery After Spinal Cord Injury In Rats By Regulating The Polarization Of Macrophages.Mol Neurobiol. 2025 Apr;62(4):4617-4630. doi: 10.1007/s12035-024-04539-0. Epub 2024 Oct 29. Mol Neurobiol. 2025. PMID: 39470871
-
Stem Cell Strategies in Promoting Neuronal Regeneration after Spinal Cord Injury: A Systematic Review.Int J Mol Sci. 2022 Oct 27;23(21):12996. doi: 10.3390/ijms232112996. Int J Mol Sci. 2022. PMID: 36361786 Free PMC article.
-
Biomaterial-Mediated Factor Delivery for Spinal Cord Injury Treatment.Biomedicines. 2022 Jul 12;10(7):1673. doi: 10.3390/biomedicines10071673. Biomedicines. 2022. PMID: 35884981 Free PMC article. Review.
-
Building-Block Size Mediates Microporous Annealed Particle Hydrogel Tube Microenvironment Following Spinal Cord Injury.Adv Healthc Mater. 2024 Oct;13(25):e2302498. doi: 10.1002/adhm.202302498. Epub 2023 Oct 5. Adv Healthc Mater. 2024. PMID: 37768019
References
-
- Abdellatif AA, Pelt JL, Benton RL, Howard RM, Tsoulfas P, Ping P, . . . Whittemore SR (2006). Gene delivery to the spinal cord: comparison between lentiviral, adenoviral, and retroviral vector delivery systems. Journal of Neuroscience Research, 84(3), 553–567. doi:10.1002/jnr.20968 - DOI - PMC - PubMed
-
- Anzalone A, Chacko J, Nishi R, Dumont C, Smith D, Shea L, . . . Anderson A (2018). Feasibility study on mouse live imaging after spinal cord injury and poly(lactide-co-glycolide) bridge implantation. Journal of Biomedical Optics, 23(6), 065007. Retrieved from 10.1117/1.JBO.23.6.065007 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

