Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination
- PMID: 33835769
- PMCID: PMC8095372
- DOI: 10.1056/NEJMoa2104840
Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination
Abstract
Background: Several cases of unusual thrombotic events and thrombocytopenia have developed after vaccination with the recombinant adenoviral vector encoding the spike protein antigen of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (ChAdOx1 nCov-19, AstraZeneca). More data were needed on the pathogenesis of this unusual clotting disorder.
Methods: We assessed the clinical and laboratory features of 11 patients in Germany and Austria in whom thrombosis or thrombocytopenia had developed after vaccination with ChAdOx1 nCov-19. We used a standard enzyme-linked immunosorbent assay to detect platelet factor 4 (PF4)-heparin antibodies and a modified (PF4-enhanced) platelet-activation test to detect platelet-activating antibodies under various reaction conditions. Included in this testing were samples from patients who had blood samples referred for investigation of vaccine-associated thrombotic events, with 28 testing positive on a screening PF4-heparin immunoassay.
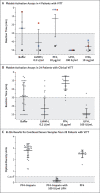
Results: Of the 11 original patients, 9 were women, with a median age of 36 years (range, 22 to 49). Beginning 5 to 16 days after vaccination, the patients presented with one or more thrombotic events, with the exception of 1 patient, who presented with fatal intracranial hemorrhage. Of the patients with one or more thrombotic events, 9 had cerebral venous thrombosis, 3 had splanchnic-vein thrombosis, 3 had pulmonary embolism, and 4 had other thromboses; of these patients, 6 died. Five patients had disseminated intravascular coagulation. None of the patients had received heparin before symptom onset. All 28 patients who tested positive for antibodies against PF4-heparin tested positive on the platelet-activation assay in the presence of PF4 independent of heparin. Platelet activation was inhibited by high levels of heparin, Fc receptor-blocking monoclonal antibody, and immune globulin (10 mg per milliliter). Additional studies with PF4 or PF4-heparin affinity purified antibodies in 2 patients confirmed PF4-dependent platelet activation.
Conclusions: Vaccination with ChAdOx1 nCov-19 can result in the rare development of immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, which clinically mimics autoimmune heparin-induced thrombocytopenia. (Funded by the German Research Foundation.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
How could a COVID vaccine cause blood clots? Scientists race to investigate.Nature. 2021 Apr;592(7854):334-335. doi: 10.1038/d41586-021-00940-0. Nature. 2021. PMID: 33846607 No abstract available.
-
SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia.N Engl J Med. 2021 Jun 10;384(23):2254-2256. doi: 10.1056/NEJMe2106315. Epub 2021 Apr 16. N Engl J Med. 2021. PMID: 33861524 Free PMC article. No abstract available.
-
Incidence of cerebral venous thrombosis and COVID-19 vaccination: possible causal effect or just chance?Eur Heart J Cardiovasc Pharmacother. 2021 Jul 23;7(4):e77-e78. doi: 10.1093/ehjcvp/pvab036. Eur Heart J Cardiovasc Pharmacother. 2021. PMID: 33930114 Free PMC article. No abstract available.
-
PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia.N Engl J Med. 2021 Jul 22;385(4):376-378. doi: 10.1056/NEJMc2106383. Epub 2021 May 19. N Engl J Med. 2021. PMID: 34010527 Free PMC article. No abstract available.
-
Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination.N Engl J Med. 2021 Jul 15;385(3):e11. doi: 10.1056/NEJMc2107227. Epub 2021 Jun 16. N Engl J Med. 2021. PMID: 34133851 No abstract available.
-
Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination.N Engl J Med. 2021 Jul 15;385(3):e11. doi: 10.1056/NEJMc2107227. Epub 2021 Jun 16. N Engl J Med. 2021. PMID: 34133852 No abstract available.
-
Suggested treatment of serious complications to COVID-19 vaccination with IdeS, a bacterial antibody-cleaving enzyme.J Thromb Haemost. 2021 Sep;19(9):2363-2364. doi: 10.1111/jth.15433. Epub 2021 Jul 9. J Thromb Haemost. 2021. PMID: 34165239 Free PMC article. No abstract available.
-
Modeling the Effects of Intravasal Administration of AstraZeneca ChAdOx1 nCoV-19 Vaccine on Human Platelets.Thromb Haemost. 2021 Dec;121(12):1681-1683. doi: 10.1055/a-1579-5308. Epub 2021 Sep 23. Thromb Haemost. 2021. PMID: 34352912 No abstract available.
-
VITT after ChAdOx1 nCoV-19 Vaccination.N Engl J Med. 2021 Dec 2;385(23):2202-2203. doi: 10.1056/NEJMc2111026. Epub 2021 Nov 3. N Engl J Med. 2021. PMID: 34731555 No abstract available.
-
VITT after ChAdOx1 nCoV-19 Vaccination.N Engl J Med. 2021 Dec 2;385(23):2203-2204. doi: 10.1056/NEJMc2111026. Epub 2021 Nov 3. N Engl J Med. 2021. PMID: 34731556 No abstract available.
References
-
- European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker (https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.ht...).
-
- Eichler P, Budde U, Haas S, et al. First workshop for detection of heparin-induced antibodies: validation of the heparin-induced platelet-activation test (HIPA) in comparison with a PF4/heparin ELISA. Thromb Haemost 1999;81:625-629. - PubMed
-
- Eekels JJM, Althaus K, Bakchoul T, et al. An international external quality assessment for laboratory diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost 2019;17:525-531. - PubMed
-
- Juhl D, Eichler P, Lubenow N, Strobel U, Wessel A, Greinacher A. Incidence and clinical significance of anti-PF4/heparin antibodies of the IgG, IgM, and IgA class in 755 consecutive patient samples referred for diagnostic testing for heparin-induced thrombocytopenia. Eur J Haematol 2006;76:420-426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
