Effects of Myo-inositol Hexaphosphate (SNF472) on Bone Mineral Density in Patients Receiving Hemodialysis: An Analysis of the Randomized, Placebo-Controlled CaLIPSO Study
- PMID: 33835939
- PMCID: PMC8259477
- DOI: 10.2215/CJN.16931020
Effects of Myo-inositol Hexaphosphate (SNF472) on Bone Mineral Density in Patients Receiving Hemodialysis: An Analysis of the Randomized, Placebo-Controlled CaLIPSO Study
Abstract
Background and objectives: In the CaLIPSO study, intravenous administration of SNF472 (300 or 600 mg) during hemodialysis significantly attenuated progression of coronary artery and aortic valve calcification. SNF472 selectively inhibits formation of hydroxyapatite, the final step in cardiovascular calcification. Because bone mineral is predominantly hydroxyapatite, we assessed changes in bone mineral density in CaLIPSO.
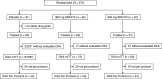
Design, setting, participants, & measurements: Patients with coronary artery calcification at screening (Agatston score of 100-3500 U) were randomized 1:1:1 to receive placebo, 300 mg SNF472, or 600 mg SNF472 as an intravenous infusion during hemodialysis three times weekly for 52 weeks. Dual-energy x-ray absorptiometry (DXA) scans were obtained at baseline (screening) and end of treatment, and between-group changes from baseline were compared using analysis of covariance.
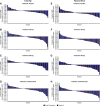
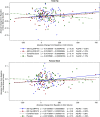
Results: Among 274 randomized patients, 202 had evaluable DXA scans at baseline and postrandomization (the DXA-modified intention-to-treat population). Mean (95% confidence interval) changes in total-hip bone mineral density from baseline to week 52 were -1.5% (-2.7% to -0.3%), -1.5% (-2.7% to -0.4%), and -2.5% (-3.8% to -1.2%) in the placebo, 300 mg SNF472, and 600 mg SNF472 groups, respectively. Mean (95% confidence interval) changes in femoral-neck bone mineral density from baseline to week 52 were -0.3% (-1.6% to 1.0%), -1.0% (-2.3% to 0.2%), and -2.6% (-4.0% to -1.3%), respectively. Regression analyses showed no correlation between change in coronary artery calcium volume and change in bone mineral density at either location. Changes in serum alkaline phosphatase, calcium, magnesium, phosphate, and intact parathyroid hormone levels were similar across treatment groups. Clinical fracture events were reported for four of 90, three of 92, and six of 91 patients in the placebo, 300 mg SNF472, and 600 mg SNF472 groups, respectively.
Conclusions: Bone mineral density decreased modestly in all groups over 1 year. In the 600 mg SNF472 group, the reduction appeared more pronounced. Reported fractures were infrequent in all groups.
Clinical trial registry name and registration number: Effect of SNF472 on Progression of Cardiovascular Calcification in End-Stage-Renal-Disease (ESRD) Patients on Hemodialysis (HD), NCT02966028.
Keywords: SNF472; bone mineral density; bone modeling and remodeling; cardiovascular; matrix mineralization.
Copyright © 2021 by the American Society of Nephrology.
Figures

References
-
- Baigent C, Burbury K, Wheeler D: Premature cardiovascular disease in chronic renal failure. Lancet 356: 147–152, 2000. - PubMed
-
- Jegatheesan D, Cho Y, Johnson DW: Clinical studies of interventions to mitigate cardiovascular risk in peritoneal dialysis patients. Semin Nephrol 38: 277–290, 2018. - PubMed
-
- Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998. - PubMed
-
- United States Renal Data System : 2018 United States Renal Data System Annual Data Report: Epidemiology of Kidney Disease in the United States National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Bethesda, MD 2018. Available at: https://www.usrds.org/media/1730/v2_c05_mortality_18_usrds.pdf. Accessed February 8, 2021
-
- Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

