Small extracellular vesicles secreted by vaginal fibroblasts exert inhibitory effect in female stress urinary incontinence through regulating the function of fibroblasts
- PMID: 33836021
- PMCID: PMC8034718
- DOI: 10.1371/journal.pone.0249977
Small extracellular vesicles secreted by vaginal fibroblasts exert inhibitory effect in female stress urinary incontinence through regulating the function of fibroblasts
Abstract
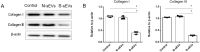
Stress urinary incontinence (SUI) is a common condition in women and associated with extra-cellular matrix (ECM) reconstruction, which is mainly regulated by fibroblasts. However, the underlying mechanism remains obscure. Small extracellular vesicles (sEVs) play fundamental biological roles in various cellular functions. Some studies suggested that the sEVs were involved in the metabolism of ECM and the function of fibroblasts. The purpose of our study was to investigate the effect of sEVs secreted by vaginal fibroblasts on the pathogenesis of SUI. We showed that the fibroblasts of female anterior vaginal wall secreted sEVs. Moreover, fibroblasts of females with SUI had significantly elevated secretion of sEVs. The collagen contents, proliferation and migration capacity of fibroblasts were decreased when fibroblasts were co-cultured with fibroblasts-derived sEVs (fibroblast-sEVs) from SUI patients. Proteomic analysis revealed that fibroblast-sEVs contained various differentially expressed proteins including TIMP2, TGF-β and ABCC4, which were involved in signaling pathways of fibroblasts regulation. Therefore, we suggested that fibroblast-sEVs contributed to the pathogenesis of SUI through various proteins including TIMP2, TGF-β and ABCC4.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Small Extracellular Vesicles Secreted by Peri-urethral Tissues Regulate Fibroblast Function and Contribute to the Pathogenesis of Female Stress Urinary Incontinence.Curr Med Sci. 2023 Aug;43(4):803-810. doi: 10.1007/s11596-023-2737-2. Epub 2023 Jul 5. Curr Med Sci. 2023. PMID: 37405606
-
Downregulation of AQP2 in the anterior vaginal wall is associated with the pathogenesis of female stress urinary incontinence.Mol Med Rep. 2017 Sep;16(3):3503-3509. doi: 10.3892/mmr.2017.7014. Epub 2017 Jul 15. Mol Med Rep. 2017. PMID: 28713996
-
Nampt promotes fibroblast extracellular matrix degradation in stress urinary incontinence by inhibiting autophagy.Bioengineered. 2022 Jan;13(1):481-495. doi: 10.1080/21655979.2021.2009417. Bioengineered. 2022. PMID: 34967693 Free PMC article.
-
Progress of mesenchymal stem cells affecting extracellular matrix metabolism in the treatment of female stress urinary incontinence.Stem Cell Res Ther. 2025 Feb 25;16(1):95. doi: 10.1186/s13287-025-04220-w. Stem Cell Res Ther. 2025. PMID: 40001265 Free PMC article. Review.
-
Puerarin protects fibroblasts against mechanical stretching injury through Nrf2/TGF-β1 signaling pathway.Int Urogynecol J. 2022 Sep;33(9):2565-2576. doi: 10.1007/s00192-022-05325-z. Epub 2022 Aug 13. Int Urogynecol J. 2022. PMID: 35962806 Review.
Cited by
-
The role of extracellular vesicles in the pathogenesis of gynecological cancer.Front Oncol. 2024 Sep 26;14:1477610. doi: 10.3389/fonc.2024.1477610. eCollection 2024. Front Oncol. 2024. PMID: 39391238 Free PMC article. Review.
-
Replacing Needle Injection by a Novel Waterjet Technology Grants Improved Muscle Cell Delivery in Target Tissues.Cell Transplant. 2022 Jan-Dec;31:9636897221080943. doi: 10.1177/09636897221080943. Cell Transplant. 2022. PMID: 35466714 Free PMC article.
-
Innovations in Stress Urinary Incontinence: A Narrative Review.Medicina (Kaunas). 2025 Jul 14;61(7):1272. doi: 10.3390/medicina61071272. Medicina (Kaunas). 2025. PMID: 40731901 Free PMC article. Review.
-
Fatty Acid Fingerprints and Hyaluronic Acid in Extracellular Vesicles from Proliferating Human Fibroblast-like Synoviocytes.Int J Mol Sci. 2022 May 17;23(10):5613. doi: 10.3390/ijms23105613. Int J Mol Sci. 2022. PMID: 35628422 Free PMC article.
-
Application of estrogen for the treatment of stress urinary incontinence in mice.Arch Gynecol Obstet. 2022 Apr;305(4):1115-1125. doi: 10.1007/s00404-022-06435-1. Epub 2022 Feb 16. Arch Gynecol Obstet. 2022. PMID: 35174407
References
-
- Tang J, Li B, Liu C, Li Y, Li Q, Wang L, et al.. Mechanism of Mechanical Trauma-Induced Extracellular Matrix Remodeling of Fibroblasts in Association with Nrf2/ARE Signaling Suppression Mediating TGF-beta1/Smad3 Signaling Inhibition. Oxid Med Cell Longev. 2017;2017:8524353. 10.1155/2017/8524353 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

